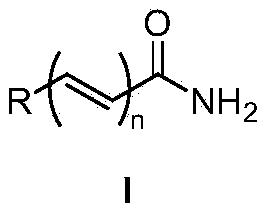
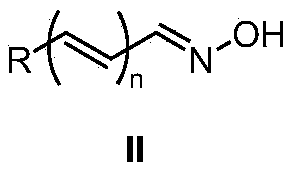
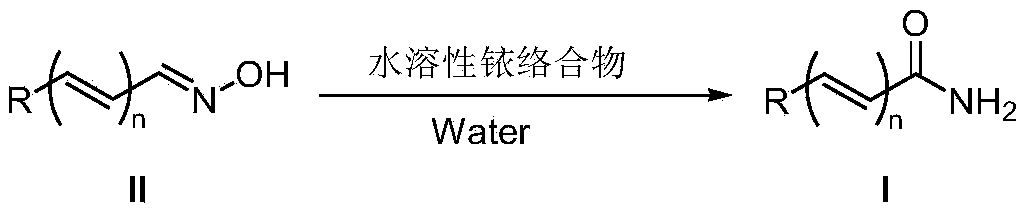
A kind of method from oxime synthetic amide
A technology for synthesizing amides and amides, applied in the formation/introduction of amides, preparation of carboxylic acid amides, chemical instruments and methods, etc., to achieve broad development prospects and low catalyst load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: benzamide
[0021] benzamide
[0022]
[0023] Benzaldehyde oxime (60.6mg, 0.5mmol), [Cp*Ir(H 2 O) 3 ][OTf] 2 (5.1mg, 0.0075mmol, 1.5mol%) and water (1ml) were sequentially added to a 25ml Schlenk reaction flask. After the reaction mixture was reacted at 110°C for 12 hours, it was cooled to room temperature, water was removed by rotary evaporation, and the target product was obtained by column separation with a yield of 89%.
[0024] 1 HNMR (500MHz, CDCl 3 )δ7.82(d,J=7.6Hz,2H,ArH),7.54(t,J=7.3Hz,1H,ArH),7.45(t,J=7.6Hz,2H,ArH),6.16(brs,1H ,NH),6.00(brs,1H,NH); 13 CNMR (125MHz, CDCl 3 )δ169.7, 133.3, 131.9, 128.6, 127.3.
Embodiment 2
[0025] Embodiment 2:4-Methylbenzamide
[0026] 4-methylbenzamide
[0027]
[0028] 4-Methylbenzaldehyde oxime (67.6mg, 0.5mmol), [Cp*Ir(H 2 O) 3 ][OTf] 2 (5.1mg, 0.0075mmol, 1.5mol%) and water (1ml) were sequentially added to a 25ml Schlenk reaction flask. After the reaction mixture was reacted at 110°C for 12 hours, it was cooled to room temperature, water was removed by rotary evaporation, and the target product was obtained by column separation with a yield of 93%. ; 1 HNMR (500MHz, CDCl 3 )δ7.71(d,J=7.9Hz,2H,ArH),7.25(d,J=7.9Hz,2H,ArH),6.12(brs,1H,NH),5.87(brs,1H,NH),2.41 (s,3H,CH 3 ); 13 CNMR (125MHz, CDCl 3 )δ169.5, 142.5, 130.4, 129.2, 127.3, 21.4.
Embodiment 3
[0029] Embodiment 3:4-isopropylbenzamide
[0030] 4-isopropylbenzamide
[0031]
[0032] 4-Isopropylbenzaldehyde oxime (81.6mg, 0.5mmol), [Cp*Ir(H 2 O) 3 ][OTf] 2 (5.1mg, 0.0075mmol, 1.5mol%) and water (1ml) were sequentially added to a 25ml Schlenk reaction flask. After the reaction mixture was reacted at 110°C for 12 hours, it was cooled to room temperature, water was removed by rotary evaporation, and the target product was obtained by column separation with a yield of 92%. mp152-153℃; 1 HNMR (500MHz, CDCl 3 )δ7.75(d,J=8.2Hz,2H,ArH),7.30(d,J=8.2Hz,2H,ArH),6.09(brs,1H,NH),5.84(brs,1H,NH),2.96 (heptet,J=6.9Hz,1H,CH),1.27(d,J=6.9Hz,6H,CH 3 ); 13 CNMR (125MHz, CDCl 3 )δ169.8, 153.2, 130.8, 127.5, 126.6, 34.1, 23.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com