Clonidine hydrochloride dry suspension and preparation method thereof
A technology of clonidine hydrochloride and dry suspension, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of low bioavailability and poor water solubility of clonidine hydrochloride, etc. , to achieve the effect of high bioavailability, fast onset of drug effect and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
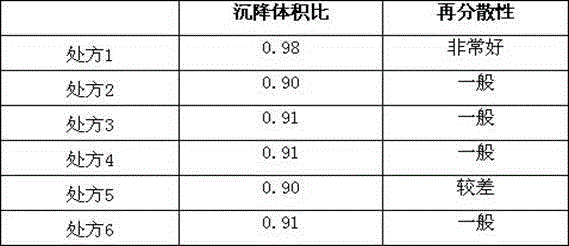
[0030] Prescription 1
[0031] Clonidine Hydrochloride 50g
[0032] Mannitol 700g
[0033] Aspartame 50g
[0034] Methylcellulose: Dextran (2:1) 50g
[0035] Anhydrous disodium hydrogen phosphate 10g
Embodiment 2
[0037] Prescription 2
[0038] Clonidine Hydrochloride 50g
[0039] Mannitol 700g
[0040] Aspartame 50g
[0041] Methylcellulose: Dextran (1:1) 50g
[0042] Anhydrous disodium hydrogen phosphate 10g
Embodiment 3
[0044] Prescription 3
[0045] Clonidine Hydrochloride 50g
[0046] Mannitol 700g
[0047] Aspartame 50g
[0048] Methylcellulose: Dextran (3:1) 50g
[0049] Anhydrous disodium hydrogen phosphate 10g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com