Influenza A virus and B virus joint detection primer, probe, kit and application
An influenza virus, combined detection technology, applied in the field of kits and applications, B type combined detection primers, influenza virus A type, probe fields, can solve the problem of reducing operation steps, etc., to reduce operation steps, improve detection efficiency, improve Detecting the effect of speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0068] Embodiment 2 tests the sensitivity of the detection method of embodiment 1
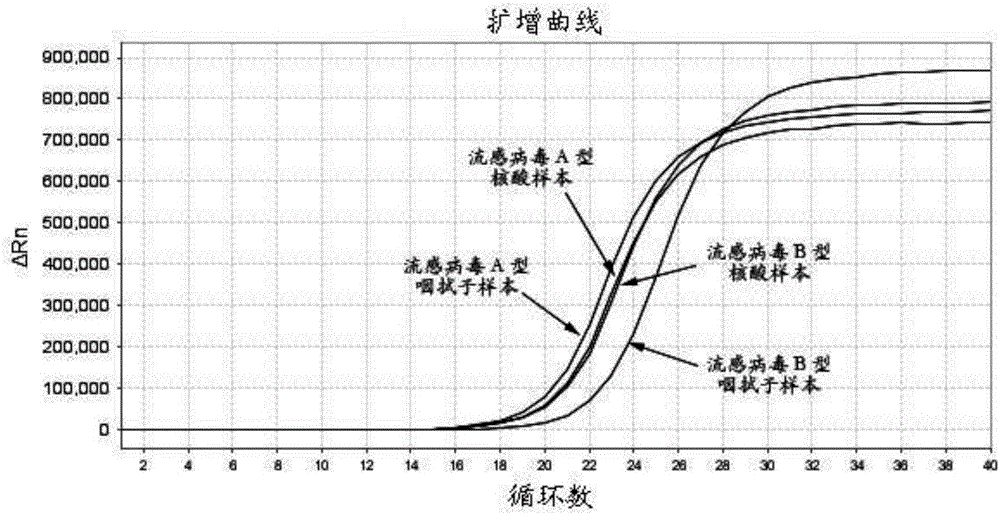
[0069] Using the optimal reaction system in step 2 and the optimal reaction conditions in step 3, perform direct amplification RT-PCR on throat swab samples and allantoic fluid samples, and obtain the amplification curve and Ct of each reaction tube from the fluorescent quantitative PCR. Values, the average Ct of each sample was calculated. From figure 1 It can be seen that for the throat swab sample, using 1 μL of purified AIV RNA as a template, the Ct value of the fluorescent quantitative PCR was 19.42 for the A-type nucleic acid sample, and 19.53 for the B-type nucleic acid sample, while the direct amplification RT of Example 1 was used. -The Ct value detected by PCR from 1 μL AIV PBS eluate is 18.93 for type A nucleic acid samples, and 21.23 for type B nucleic acid samples (such as figure 1 ).
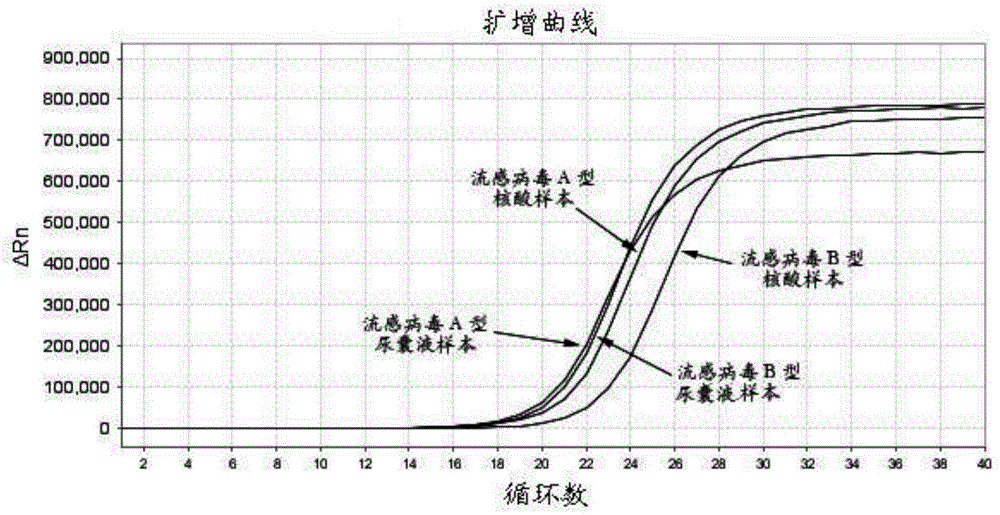
[0070] For allantoic fluid samples, using 1 μL of purified AIV RNA as a template, the Ct valu...
Embodiment 3
[0071] Embodiment 3 tests the tolerance of throat swab fluid of the detection method of embodiment 1
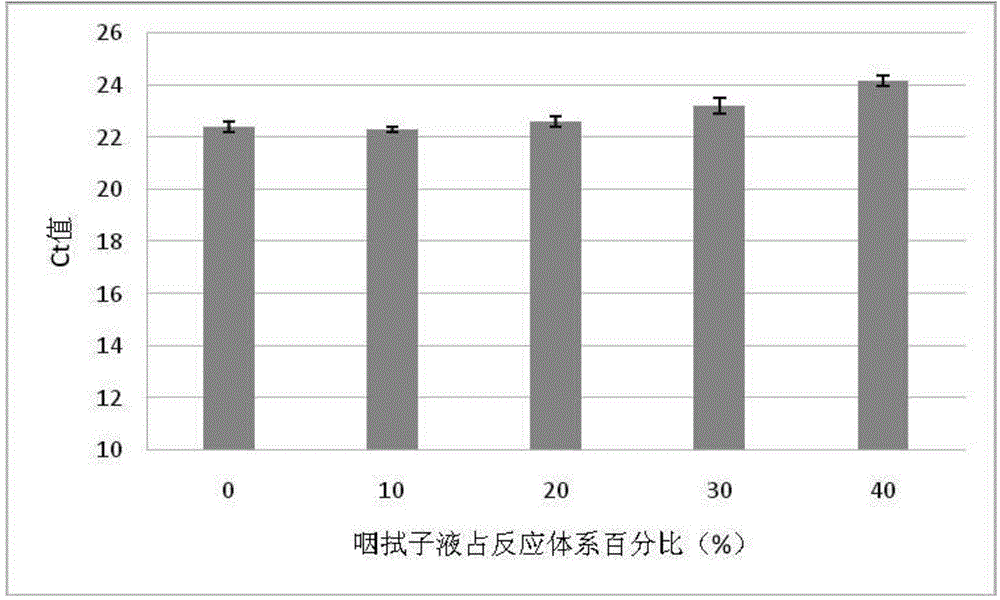
[0072]According to the optimal reaction system in step 2 of Example 1, the reaction solution except the throat swab solution was prepared; 1 μL AIV positive throat swab solution was diluted to 2.5, 5, 7.5, respectively with the negative throat swab solution not containing AIV. 10 μL were added to the reaction tubes to keep the AIV virus copy numbers in each tube consistent, and the percentages of throat swab fluid in the total volume were 10%, 20%, 30%, and 40%, respectively. Put each reaction tube into a fluorescent quantitative PCR instrument, and perform the reaction according to the optimal reaction conditions in Step 3 of Example 1.
[0073] The result is as image 3 As shown, the direct amplification RT-PCR still has high activity in up to 40% throat swab fluid, and the change of Ct value is less than 2 values compared with 10% concentration, which has high tolerance...
Embodiment 4
[0074] Embodiment 4 Test the detection dynamic range and the linearity of the detection method of embodiment 1
[0075] According to the optimal reaction system in step 2 of Example 1 to prepare the reaction solution except for the sample; take a positive sample of AIV throat swab and dilute it with a negative throat swab that does not contain AIV to obtain a 10-fold gradient of positive Throat swab fluid sample 10 0 ,10 -1 ,10 -2 ,10 -3 , all reaction tubes maintain a final concentration of 5% of the throat swab fluid. Take 10 μL of throat swab liquid of various dilutions and add them to reaction tubes, place them in a fluorescent quantitative PCR instrument, and perform the reaction according to the optimal reaction conditions in Step 3 of Example 1.
[0076] The results showed that the pharyngeal swab liquid of 1000 times dilution still obtained positive detection signal ( Figure 4 Fluorescent PCR curves for each concentration, Figure 5 is a linear range graph); the...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap