A pharmaceutical composition for preventing and treating chronic hepatitis B and its application
A technology for chronic hepatitis B and composition, applied in the directions of drug combination, drug delivery, active ingredients of heterocyclic compounds, etc., can solve problems such as large toxic and side effects, and achieve the effects of low toxic and side effects, avoiding degradation and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Culture of human liver cancer cell line HepG2.2.15 transfected with HBV
[0014] After thawing the HepG 2.2.15 cells transfected with HBV, inoculate into 25cm 2 Culture flask, the concentration is 1×10 5 cells / mL, after the cells are confluent, add 0.25% (w / v) trypsin solution to digest at 37°C for 3-5min. Add culture medium by pipetting, subculture at 1:3, collect supernatant every day for 2 consecutive weeks, and refrigerate for inspection. The cell culture medium is MEN medium, every 100mL contains 10mL of fetal bovine serum, 1mL of 3% (w / v) glutamine solution, G418 380μg / mL, gentamicin 50U / mL; cell digestion solution is 0.25% ( w / v) trypsin solution, prepared with Hanks solution.
Embodiment 2
[0015] Embodiment 2: Toxicity test of theanine bromide to HepG2.2.15 cells
[0016] Get the logarithmic growth phase HepG2.2.15 cells cultured in Example 1, adjust the cell concentration with cell culture medium to be 1 × 10 5 mL -1 , seed the cells in a 96-well plate, 200 μL per well, 37 °C 5% CO 2 Cultivate in an incubator, and discard the original culture medium after it adheres to the wall. Add 200 μL of culture solution with theanine bromide concentration of 16, 64, 125, 250, 500 μmol / L to each well of the theanine bromide group respectively, and set the cell culture wells without drug addition as the blank control group , each group repeated 6 wells. Place each group of cells in CO 2 Incubate in an incubator for 72 hours, add 10 μL of 5 μg / mL MTT to each well of cells, keep 100 μL of supernatant in each well, and store at 37°C in 5% CO 2 After culturing in the incubator for 4 hours, measure the optical density (OD) value of each well with a microplate reader at a re...
Embodiment 3
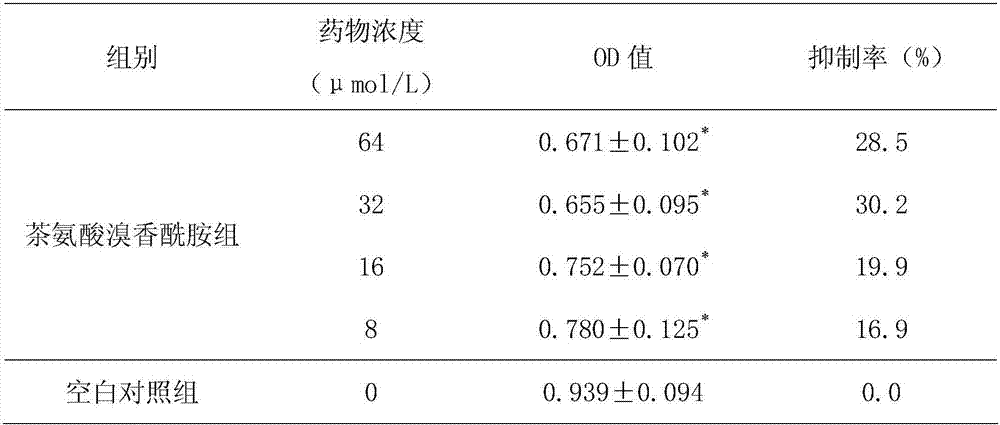
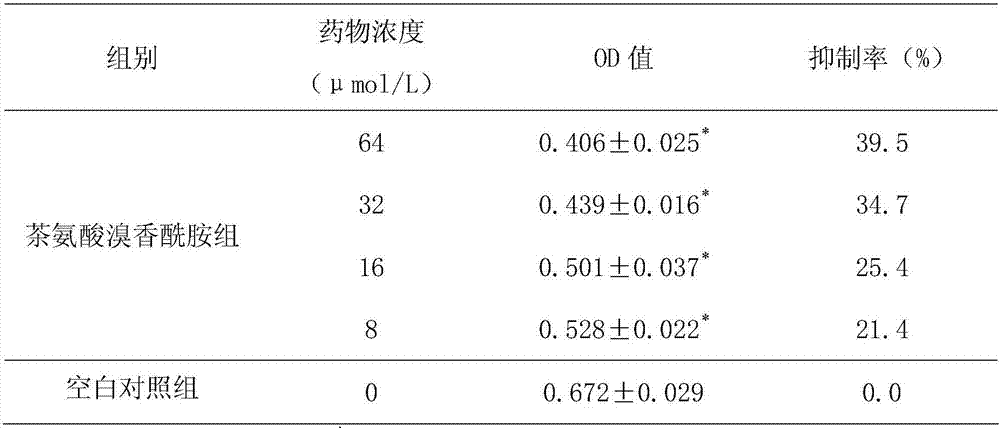
[0017] Embodiment 3: The inhibitory test of theanine bromide aromatic amide to HepG2.2.15 cell secretion HBsAg and HBeAg
[0018] Get the logarithmic growth phase HepG2.2.15 cells cultured in Example 1, adjust the cell concentration with cell culture medium to be 1 × 10 5 mL -1 , seed the cells in a 96-well plate, 200 μL per well, 37 °C 5% CO 2 Cultivate in an incubator, and discard the original culture medium after it adheres to the wall. In each well of the theanine bromide group with different doses, 200 μL of culture solution with theanine bromide concentration of 8, 16, 32, and 64 μmol / L was added respectively. Groups were replicated for 6 wells. Place each group of cells in CO 2 After culturing in the incubator for 72 hours, the supernatant of cell culture in each group was collected for enzyme-linked immunosorbent assay. The determination method was carried out according to the operating instructions of HBeAg kit and HBsAg kit (Huamei Bioengineering Co., Ltd.). Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com