Bispecific asymmetric heterodimers comprising anti-cd3 constructs
A technology of heterodimers and constructs, applied in the direction of antibodies, antibody medical ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0323] Example 1: Bispecific CD3-CD19 scFv fused to asymmetric IgGl Fc.
[0324] A bispecific CD3-CD19 scFv fused to an asymmetric IgG1 Fc heterodimer exhibiting stability comparable to native Fc homodimers is a novel composition identified as v873. v873 belongs to a novel family of CD3-based bispecific asymmetric IgGl antibodies that can be expressed and purified in mammalian CHO cells in significantly higher yields compared to the Amgen / Micromet bscCD19xCD3 BiTE bispecific. V873 exhibits unexpected effector cell: target cell binding, bridging, and target cell killing.
[0325] V873 and CD3-based bispecific asymmetric antibodies have utility in targeting T cell-mediated killing of diseased cells and thus may be applicable in the treatment of cancer as well as autoimmune and inflammatory diseases. v873 is a bispecific CD3-CD19 scFv fused to an asymmetric IgG1 Fc. v873 represents a novel class of bispecific asymmetric antibodies comprising an anti-CD3 warhead and a second war...
Embodiment 2
[0331] Example 2. Design, expression and purification of heteromultimeric constructs with heterodimeric Fc.
[0332] Exemplary bispecific anti-CD3 and anti-CD19 heterodimeric antibodies
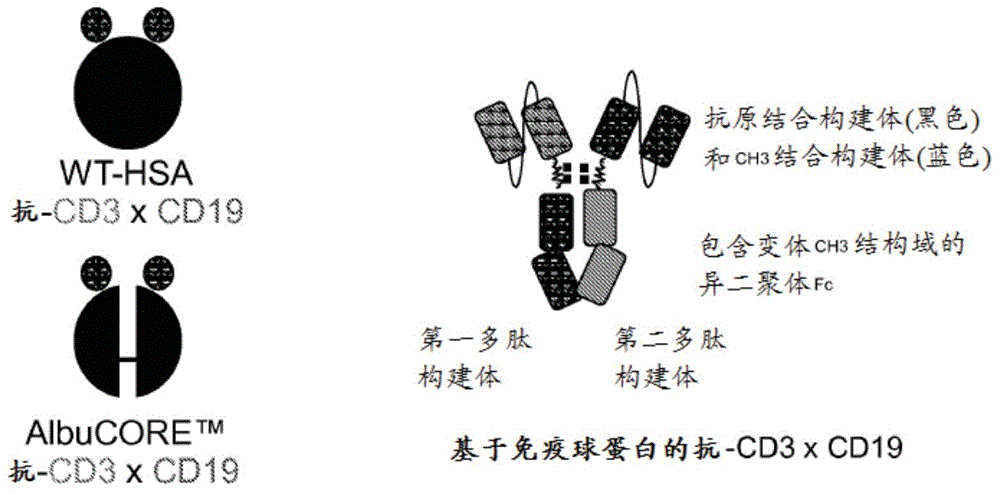
[0333] An exemplary schematic representation of an anti-CD3 / anti-CD19 antibody is shown in Figure 1A.
[0334] v873, v874, v875 exemplify bispecific anti-CD3 / anti-CD19 heterodimeric Fc constructs and were prepared and tested as described below. When the description includes a reference to a BiTE, it means that the antibody construct has the same properties as the anti- The same amino acid sequence as the VH or VL of the CD3 anti-CD19 BiTE molecule.
[0335] v873 has an anti-CD19 BiTE(VL-VH) scFv on chain A of the heterodimeric Fc and a CD3 BiTE on chain B TM (VH-VL) scFv with mutation L351Y_F405A_Y407V on chain A and mutation T366L_K392M_T394W on chain B. [The polypeptide sequence corresponds to SEQ ID No: 26 and 28]
[0336] v874 has an anti-CD19 BiTE on chain A of the heterodimeric Fc...
Embodiment 3
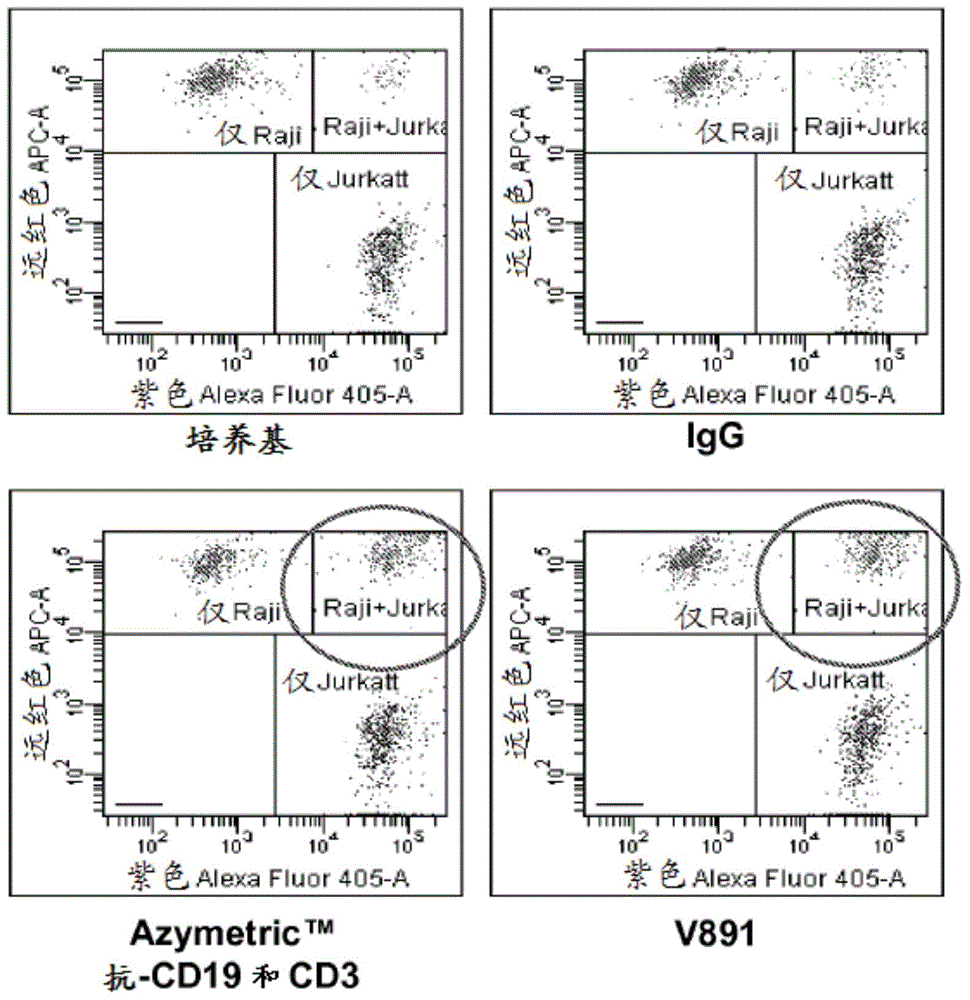
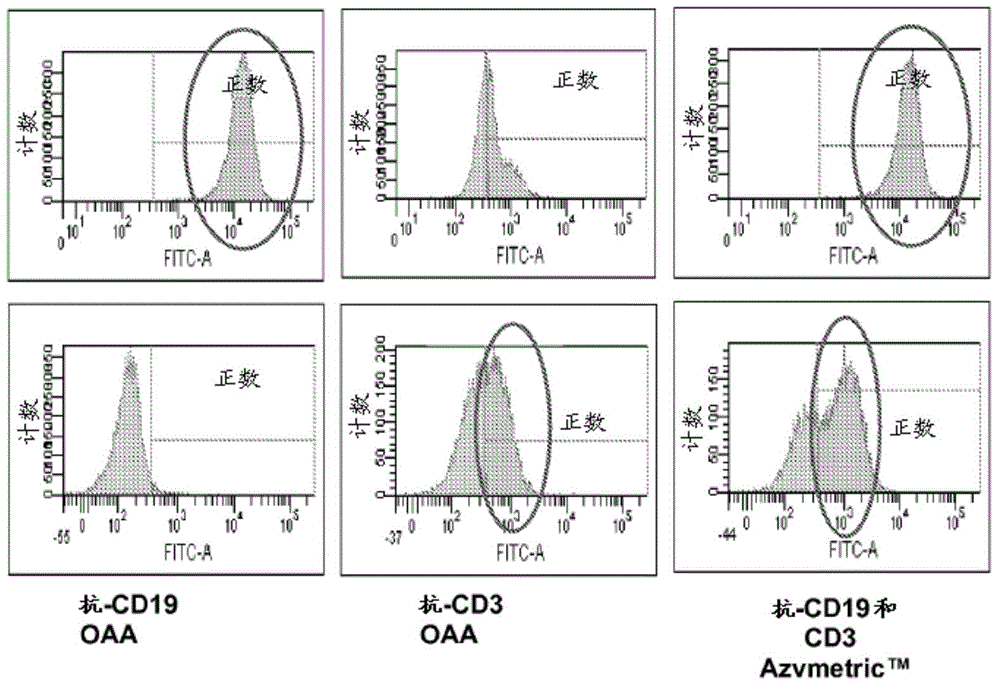
[0407] Example 3: Heteromultimeric v873 can bridge Jurkat CD3 T cells and Raji CD19 B cells.
[0408] The ability of v873 to bridge T cells and B cells was tested by FACS analysis as follows.
[0409] Whole-cell bridging by FACS
[0410] 1x10 that will be suspended in RPMI 6 Cells / ml were labeled and mixed with 0.3 μM of the appropriate CellTrace marker and incubated for 25 min at 37 °C in a water bath.
[0411] The pellet was resuspended in 2ml of L10+GS1+NaN3 to a final concentration of 5x x106 cells / ml.
[0412] Cell suspensions were analyzed by flow cytometry (1 / 5 dilution) to verify proper cell labeling and laser settings. flow-check and flow-set fluorophores for verification of instrument standardization, optical alignment and fluidics
[0413] After validation by flow cytometry and prior to bridging, each cell line was split at the desired ratio at 1x106 cells / ml at a final concentration.
[0414] T:T bridging was scored with Jurkat-violet+Jurkat-far red, B:B bridg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com