Bovine Foot and Mouth Disease Type A Polypeptide Vaccine
A technology of bovine foot-and-mouth disease and polypeptide vaccines, applied in the field of medicine, can solve the problems that affect the use of new vaccines, cannot effectively protect animals, and have poor effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1, the solid-phase synthesis of bovine foot-and-mouth disease type A polypeptide vaccine polypeptide
[0075] The present invention studies the variation of the main antigenic sites of the FMD by determining the sequence of the newly popular strains of the domestic FMD and combining the sequences of the FMD vaccine strains, and counts the variation frequency of the main mutated amino acid sites, and at the same time combines computer-aided FMD Analysis and prediction of antigenic sites, chemical synthesis of possible antigenic site peptides, that is, according to the statistical variation frequency of variable sites, different amino acids are used at these sites to obtain a multiplicity covering all current possible variable sites A candidate polypeptide antigen. Furthermore, these candidate polypeptide antigens were screened through a large number of animal experiments, and the polypeptide antigens capable of inducing an immune response in animals with a hi...
Embodiment 2
[0119] Embodiment 2, the preparation of bovine foot-and-mouth disease type A polypeptide vaccine
[0120] 1.1 Preparation of antigen aqueous phase
[0121] Weigh the synthetic peptide antigens shown in Polypeptide 1, Polypeptide 2, Polypeptide 3 or Polypeptide 4 prepared according to Example 1, mix them according to the molar ratio of the antigens at 1:1:1:1, and then use sterile water for injection to dissolve the synthetic peptide antigens The total antigen concentration was diluted to 50 μg / ml. The resulting antigen solution was filtered through a filter with a pore size of 0.2 μm and sterilized.
[0122] 1.2 Preparation of oil phase adjuvant
[0123] Sterilize the oil phase adjuvant at 50V at 121°C for 30min, and set aside.
[0124] 1.3 Emulsion of synthetic peptide vaccine
[0125] Clean the IKA emulsification equipment with 2000ml of sterilized distilled water for 3 times, then put the oil phase into the emulsification tank at 20-28°C according to the volume ratio ...
Embodiment 3
[0127] Embodiment 3, the effect test of bovine foot-and-mouth disease type A polypeptide vaccine
[0128] 1. Materials and methods
[0129] 1.1 Materials
[0130] 1.1.1 Synthetic peptide vaccines
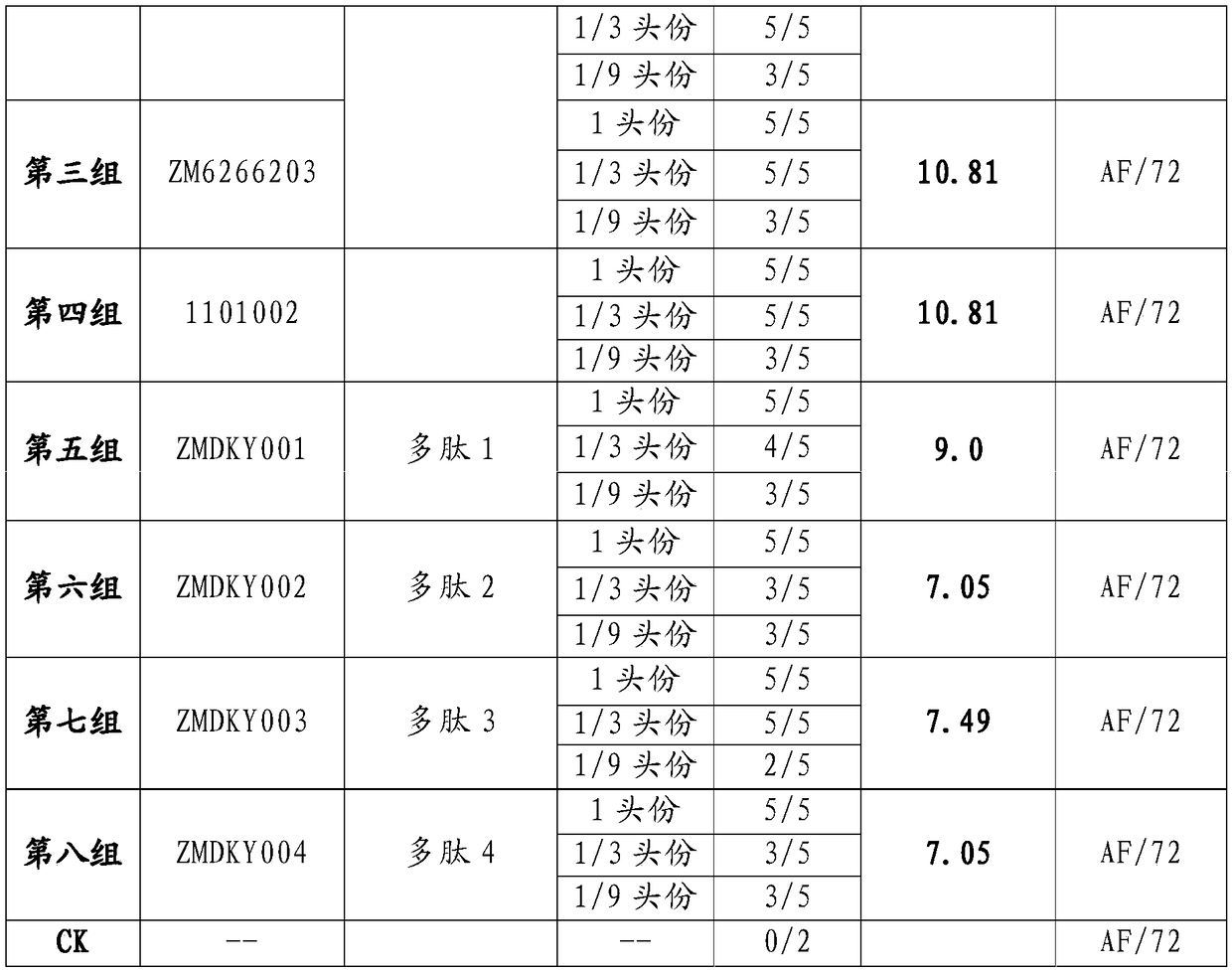
[0131] According to Example 1, polypeptide antigens with sequences such as Polypeptide 1, Polypeptide 2, Polypeptide 3 or Polypeptide 4 were prepared, and then according to Example 2, the A-type foot-and-mouth disease synthetic peptides containing Polypeptide 1, Polypeptide 2, Polypeptide 3 and Polypeptide 4 mixed components were prepared. Vaccines, the corresponding batch numbers are: ZM6266201, ZM6266202, ZM6266203. Simultaneously, according to the method of Example 2, polypeptide 1, polypeptide 2, polypeptide 3 or polypeptide 4 are respectively one-component foot-and-mouth disease type A polypeptide vaccines that are antigens, and the corresponding batch numbers are: ZMDKY001, ZMDKY002, ZMDKY003, ZMDKY004.
[0132] 1.1.2 Test animals
[0133] 122 6-month-old healthy yellow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com