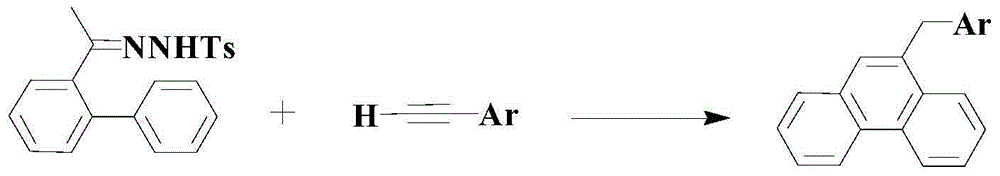
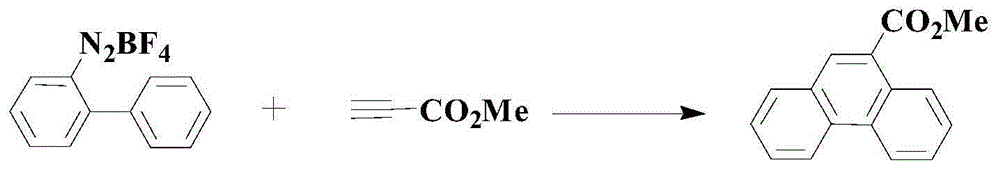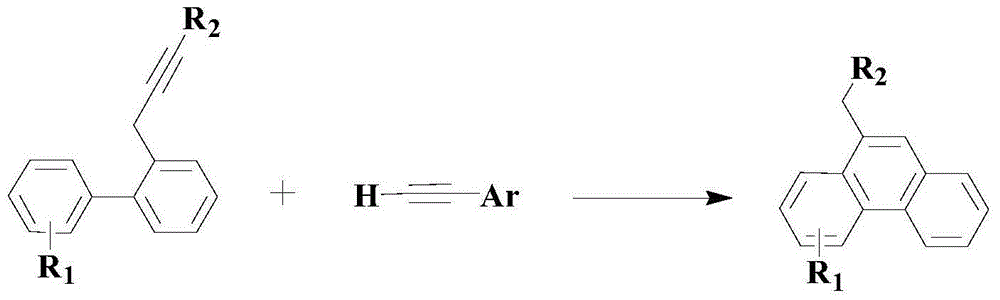Method for synthesizing medical intermediate phenanthrene compound
A synthesis method and compound technology, which are applied in the synthesis of fused ring compounds and the synthesis of pharmaceutical intermediates phenanthrene compounds, can solve problems such as low production efficiency, insufficient utilization of raw materials, and inability to meet production requirements, and achieve high yield and wide The effect of industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] Add an appropriate amount of mixed solvent consisting of PEG-400 and 1-allyl-3-methylimidazolium tetrafluoroborate (the volume ratio of the two is 1:0.1) to the reactor, and then replace it twice with nitrogen, so that The reactor is a nitrogen atmosphere; then add 100mmol of the above formula (II) compound 2-bromo-4'-chlorobiphenyl, 200mmol of the above formula (III) compound styrene, by 3mmol PdCl 2 (dppf) and 6 mmol hexafluorophosphate tetraacetonitrile copper composite catalyst, 10 mmol ligand L1 and 200 mmol diisopropylethanolamine, heated to 60 ° C under stirring, and reacted at this temperature for 12 hours.
[0048] After the reaction, add deionized water to the reaction system, fully shake and wash, separate the organic layer, wash with deionized water again, separate the organic layer; concentrate the organic layer under reduced pressure, remove, and put the residue on a silica gel column Chromatography, eluting with a mixed solvent of n-hexanol an...
Embodiment 2
[0051]
[0052] Add an appropriate amount of mixed solvent (the volume ratio of the two is 1:0.2) consisting of PEG-400 and 1-allyl-3-methylimidazolium tetrafluoroborate to the reactor, and then replace it twice with nitrogen, so that The reactor is a nitrogen atmosphere; then add 100mmol of formula (II) compound 2-bromobiphenyl, 300mmol of formula (III) compound 1-methyl-3-vinylbenzene, 3mmol PdCl 2 (dppf) and 9 mmol hexafluorophosphate tetraacetonitrile copper composite catalyst, 15 mmol ligand L1 and 250 mmol diisopropylethanolamine, heated to 70 ° C under stirring, and reacted at this temperature for 10 hours.
[0053] After the reaction, add deionized water to the reaction system, fully shake and wash, separate the organic layer, wash with deionized water again, separate the organic layer; concentrate the organic layer under reduced pressure, remove, and put the residue on a silica gel column Chromatography, eluting with a mixed solvent of n-hexanol and chloroform with...
Embodiment 3
[0056]
[0057] Add an appropriate amount of mixed solvent (the volume ratio of the two is 1:0.3) consisting of PEG-400 and 1-allyl-3-methylimidazolium tetrafluoroborate to the reactor, and then replace it twice with nitrogen, so that The reactor is a nitrogen atmosphere; then add 100mmol of the formula (II) compound 2-bromobiphenyl, 400mmol of the formula (III) compound 1-vinylnaphthalene, by 3mmol PdCl 2 (dppf) and 12 mmol hexafluorophosphate tetraacetonitrile copper composite catalyst, 20 mmol ligand L1 and 300 mmol diisopropylethanolamine, heated to 80 ° C under stirring, and reacted at this temperature for 8 hours.
[0058] After the reaction, add deionized water to the reaction system, fully shake and wash, separate the organic layer, wash with deionized water again, separate the organic layer; concentrate the organic layer under reduced pressure, remove, and put the residue on a silica gel column Chromatography, the mixed solvent of n-hexanol and chloroform with a vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com