A kind of 2,1,3-benzothiadiazole derivative and its preparation method and application
A technology of benzothiadiazole and its derivatives, which is applied in the field of 2,1,3-benzothiadiazole derivatives and its preparation, can solve problems such as inconsistent relative molecular weight and unstable properties, and achieve improved photoelectric conversion efficiency , high electron affinity, stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] In the second aspect, the embodiment of the present invention provides a preparation method of 2,1,3-benzothiadiazole derivatives, comprising the following steps:
[0057] Provide chemical structural formulas such as the compound shown in formula A and the compound shown in formula B respectively,
[0058] A: B:
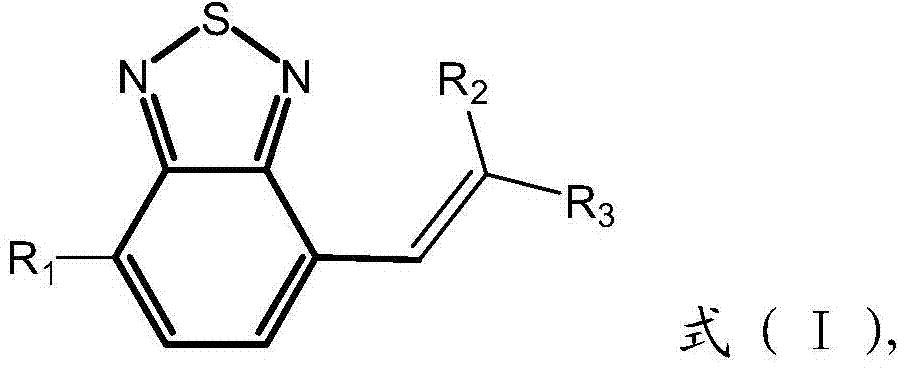
[0059] Among them, R 1 is an electron-rich substituent, R 2 and R 3 where one is an electron deficient substituent and the other is a hydrogen atom, or R 2 and R 3 Both of them are electron deficient substituents;
[0060] In an oxygen-free environment, the compound shown in formula A and the compound shown in formula B with a molar ratio of 1:1 to 1:10 are added into an organic solvent containing a catalyst for dissolution to obtain a mixed solution, and the The above mixed solution is subjected to Knaevengel condensation reaction at 40°C to 150°C, and the reaction time is 4 to 48 hours to obtain the product, and the organic solvent is acetonitrile,...
Embodiment 1
[0129] A preparation method of 2,1,3-benzothiadiazole derivatives, comprising the following steps:
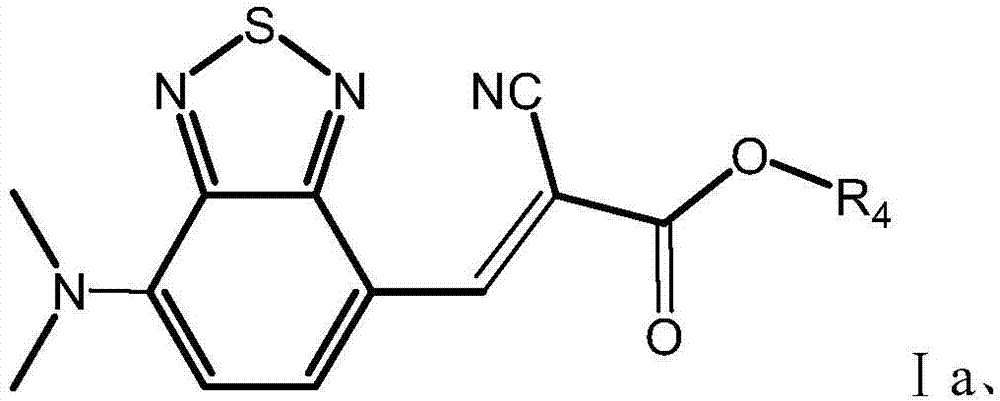
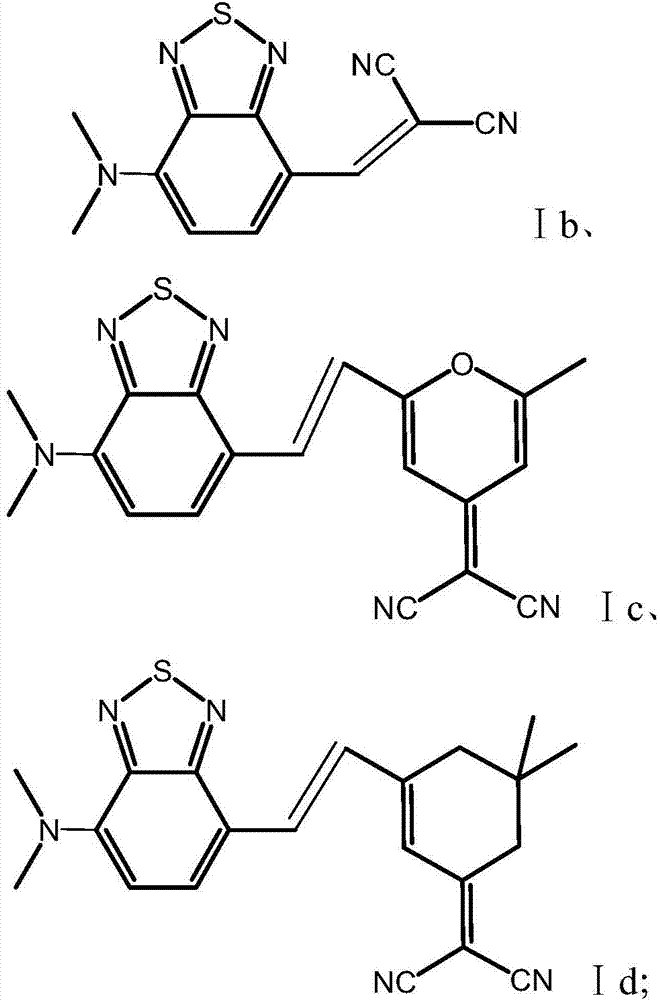
[0130] 4-formyl-7-dimethylaminobenzothiadiazole with chemical structural formula shown in formula A1 and cyanoacetoxypropyl ester shown in formula a1 are dissolved in acetonitrile containing catalyst piperidine in equimolar ratio , to obtain a mixed solution, the concentration of 4-formyl-7-dimethylaminobenzothiadiazole in the mixed solution is 20mg / ml, and the molar weight of piperidine is 4-formyl-7-dimethylaminobenzothiadiazole 2% of the molar amount of oxadiazole; under the state of nitrogen protection, the mixed solution was stirred at 80°C, and the 4-formyl-7-dimethylaminobenzothiadiazole and cyanoacetate alkyl ester in the mixed solution produced croven The product was obtained by the Gehl condensation reaction with a reaction time of 4 hours. The product is purified by silica gel column chromatography to obtain 2,1,3-benzothiadiazole derivatives shown in Ia1, and the r...
Embodiment 2
[0137] A preparation method of 2,1,3-benzothiadiazole derivatives, comprising the following steps:
[0138] Dissolve 4-formyl-7-dimethylaminobenzothiadiazole with chemical structural formula as shown in formula A1 and cyanoacetate hexyl ester with chemical structural formula as shown in formula a2 in a molar ratio of 1:10 in a catalyst containing alkaline In the toluene of aluminum oxide, a mixed solution is obtained, the concentration of 4-formyl-7-dimethylaminobenzothiadiazole in the mixed solution is 50 mg / ml, and the molar weight of basic aluminum oxide is 4- 10% of the molar weight of aldehyde-7-dimethylaminobenzothiadiazole; under the state of nitrogen protection, the mixed solution was stirred at 40°C, and the 4-aldehyde-7-dimethylaminobenzothiadiazole in the mixed solution The product was obtained by Knaveningel condensation reaction with alkyl cyanoacetate, and the reaction time was 24 hours. The product is purified by silica gel column chromatography to obtain 2,1,3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com