Optically pure itraconazole key intermediate, synthetic method thereof, and method for synthesizing optically pure itraconazole from the intermediate
A technology for itraconazole and intermediates, applied in the field of synthesis of optically pure itraconazole, which can solve the problems of high production cost, difficulty in industrialization, and complex components of glycerol tosylate, so as to reduce prices and production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of Compound Ⅶ-a
[0037]
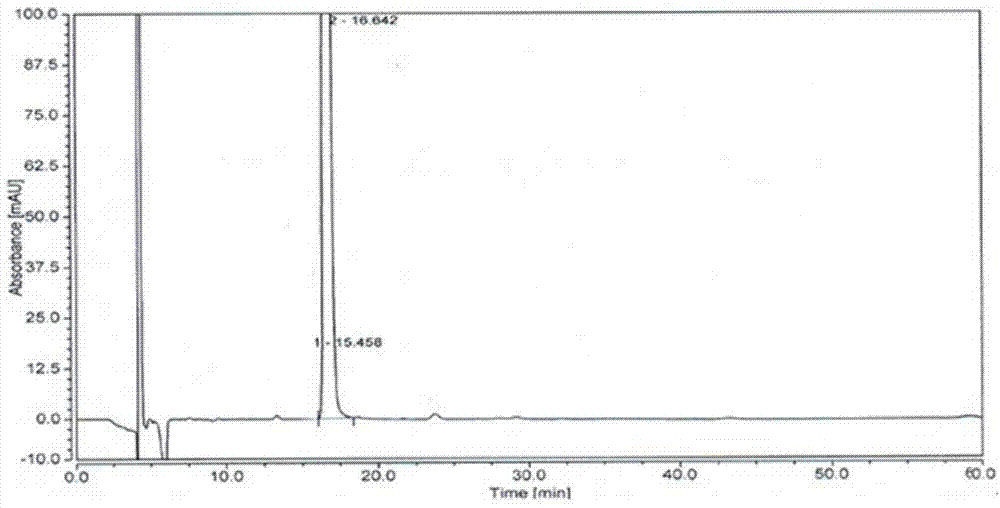
[0038]In a 200ml three-necked flask, add compound I (9.50g, 37mmol, 1.0eq), S-3-chloro-1,2-propanediol (8.20g, 74mmol, 2.0eq) and 80mL of toluene, and add three Fluoromethanesulfonic acid (22g, 5.0mmol, 4.0eq), after the dropwise addition, heat to 80°C, stir and react for 6h, add 100ml of saturated sodium bicarbonate solution to the reaction solution, stir for 30min, separate the liquids, and collect the organic phase , and concentrated under reduced pressure to obtain 15 g of optically pure compound VII-a (yellow oil, S, S-configuration), with a purity of 86% and a yield of 99.7%.
[0039] 1 HNMR (400MHz, CDCl 3 ,):
[0040] 8.13(s,1H),7.77(s,1H),7.42~7.44(d,H,J=8.0),7.35(s,1H),7.14~7.16(d,1H,J=8.0),4.63~4.75 (m,2H,J=16.0),4.16(m,1H),3.69~3.78(m,2H),2.88~2.93,3.23~3.26(m,2H)
[0041] ESI-MS: 348.0 (M+H + ).
Embodiment 2
[0043] Synthesis of Compound Ⅶ-a
[0044]
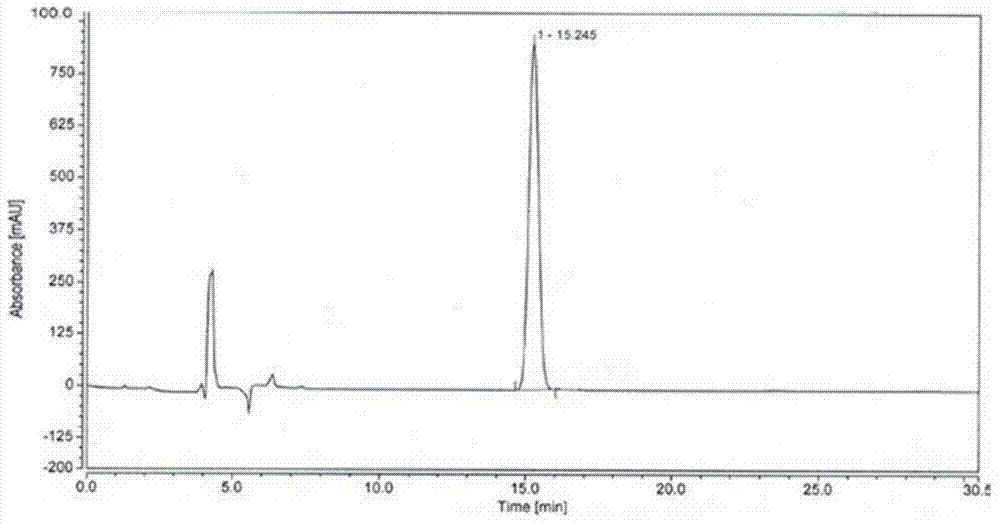
[0045] In a 500ml three-necked flask, add compound I (9.50g, 37mmol, 1.0eq), S-3-chloro-1,2-propanediol (16g, 148mmol, 4eq) and toluene 90mL, and add p-toluenesulfonic acid while stirring (14g, 74mmol, 2eq), after the dropwise addition, heated to 100°C, stirred and reacted for 12h, added 150ml of saturated sodium bicarbonate solution to the reaction solution, stirred for 30min, separated, collected the organic phase, and concentrated under reduced pressure to obtain 12g of optically pure compound VII (yellow oil, S,S-configuration), purity 84%, yield 78.1%.
Embodiment 3
[0047] Synthesis of Compound Ⅶ-b
[0048]
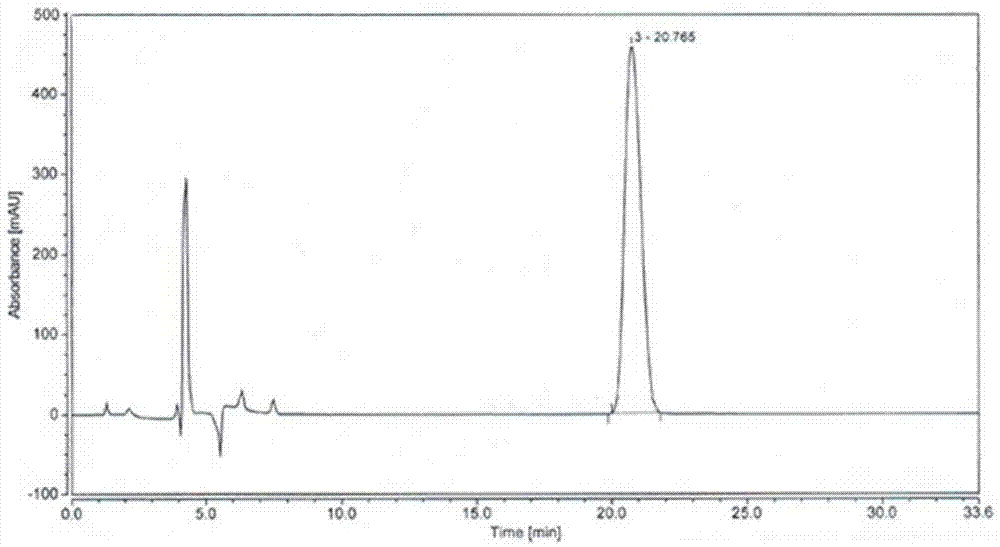
[0049] In a 500ml three-necked flask, add compound I (9.50g, 37mmol, 1.0eq), R-3-chloro-1,2-propanediol (20.5g, 185mmol, 5eq) and 90mL of toluene, and add methanesulfonate dropwise while stirring Acid (28g, 296mmol, 8eq), after the dropwise addition, heated to 40°C, stirred and reacted for 4h, added 200ml of saturated sodium bicarbonate solution to the reaction solution, stirred for 30min, separated, collected the organic phase, concentrated under reduced pressure, 14 g of optically pure compound VII (yellow oil, S,S-configuration) was obtained with a purity of 83% and a yield of 90%.
[0050] 1 HNMR (400MHz, CDCl 3 ,):
[0051] 7.98(s,1H),7.58(s,1H),7.25~7.28(d,H,J=12.0),7.17(s,1H),6.97~6.99(d,1H,J=8.0),4.47~4.63 (m,2H,J=16.0),3.98(m,1H),3.51~3.60(m,2H),2.73~2.78,3.06~3.08(m,2H)
[0052] ESI-MS: 348.1 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com