Method for acquiring medicinally-advantageous crystal form of tolbutamide through rapid cooling and crystallization
A technology of rapid cooling and glycolysis, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as environmental protection and complicated operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] A method for obtaining a medicinally advantageous crystal form of tolamide by rapid cooling and crystallization, comprising the steps of:
[0015] (1) Prepare a supersaturated tolamide solution: add 0.48 g of tolamide I crystal form to 15 ml of ethanol aqueous solution with a volume concentration of 66.7%, and ultrasonically dissolve at 30°C for 20 minutes; use an organic filter with a pore size of 0.22 μm Membrane filtration to remove insoluble matter;
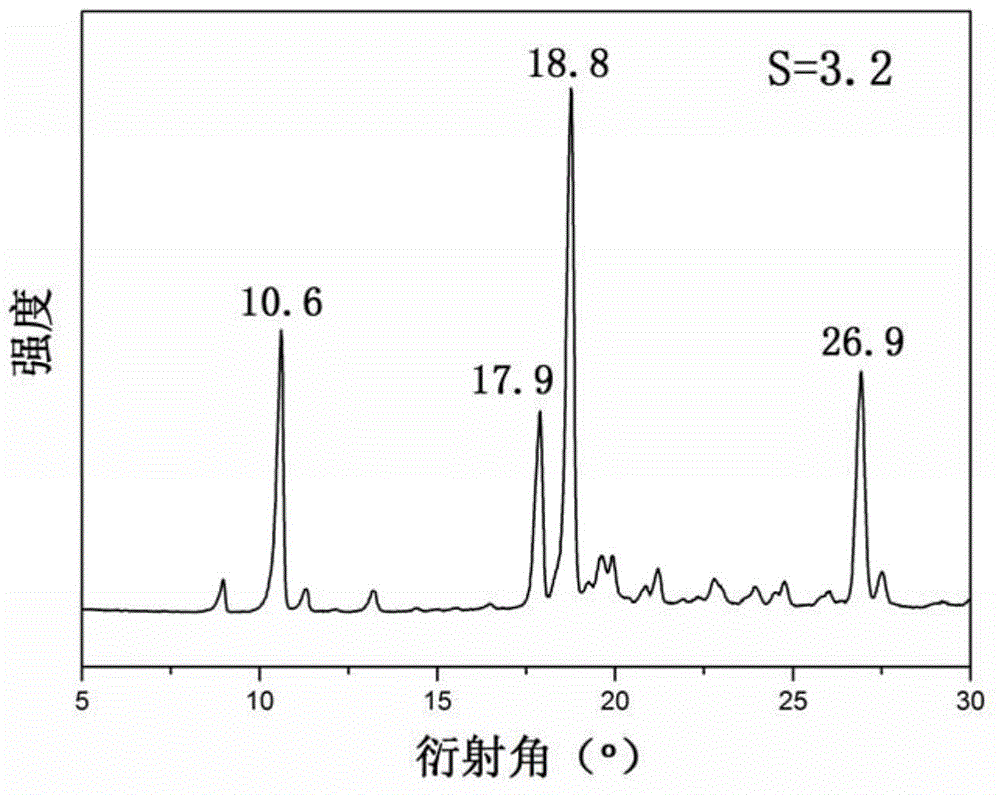
[0016] (2) Put the filtered supersaturated tolamide solution in a crystallization bottle, seal it with a sealing film and make a small hole so that the pressure inside and outside the crystallization bottle is the same; keep the temperature at 30°C for 13 minutes, then quickly cool down to 0 °C, the cooling rate was controlled at 13 °C / min, and the medicinally advantageous crystal form IV of Jiatangning was obtained. Use XRD to investigate the crystal polymorphism obtained in the solution, such as figure 1 shown.
...
Embodiment 2
[0019] (1) Prepare a supersaturated tolamide solution: add 0.53 g of tolamide I crystal form in proportion to 15 ml of ethanol aqueous solution with a volume concentration of 60%, and ultrasonically dissolve at 30°C for 30 minutes; use a 0.22 μm pore size Organic membrane filtration to remove insoluble matter;
[0020] (2) Put the filtered supersaturated tolamide solution in a crystallization bottle, seal it with a sealing film and pierce a small hole so that the pressure inside and outside the crystallization bottle is the same; keep it at a constant temperature of 30°C for 10 minutes, then quickly cool down to -5 °C, the cooling rate was controlled at 10 °C / min, and the pharmaceutically advantageous crystal form IV of Jiatangning was obtained. The crystal polymorphs obtained in the solution were investigated by XRD, and the crystal form IV of togatonin was also obtained.
Embodiment 3
[0022] (1) Prepare a supersaturated tolamide solution: add 0.50 g of tolamide I crystal form to 15 ml of ethanol aqueous solution with a volume concentration of 70% in proportion, and ultrasonically dissolve at 35°C for 20 minutes; Organic membrane filtration to remove insoluble matter;
[0023] (2) Put the filtered supersaturated tolamide solution in a crystallization bottle, seal it with a sealing film and make a small hole so that the pressure inside and outside the crystallization bottle is the same; keep the temperature at 30°C for 15 minutes, then quickly cool down to 4°C , the cooling rate was controlled at 15°C / min, and the pharmaceutically advantageous crystal form IV of Jiatangning was obtained. The crystal polymorphs obtained in the solution were investigated by XRD, and the crystal form IV of togatonin was also obtained.
[0024] The invention was funded by the National Natural Science Foundation of China (project number: 21106094)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com