P16INK4A derived peptides for prophylaxis and therapy of HPV-associated tumors and other p16 expressing tumors
A p16ink4, tumor technology, applied in the fields of peptide sources, specific peptides, antibody medical components, etc., can solve problems such as no therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] For the preparation of peptides showing a specific degree of identity, eg, genetic engineering can be used to introduce amino acid changes at specific positions in the cloned DNA sequence to identify regions important for peptide function. For example, site-directed mutagenesis or alanine scanning mutagenesis (introducing a single alanine mutation at each residue in the molecule) can be used (Cunningham and Wells, 1989). The resulting mutant molecules can then be tested for immunogenicity using the assays in the Examples.
[0035] Preferably, the variant is characterized by no more than 8 amino acids, more preferably no more than 6 amino acids, even more preferably no more than 4 amino acid substitutions, deletions and / or additions.
[0036] In raw p16 Ink4a - Fragments In fragments, at least 5 contiguous amino acids, preferably at least 10 contiguous amino acids, more preferably at least 15 contiguous amino acids, even more preferably at least 20 contiguous amino acid...
Embodiment 1
[0050] Targeting p16 in patients with HPV-associated neoplasia INK4a Peptide T cell reactivity
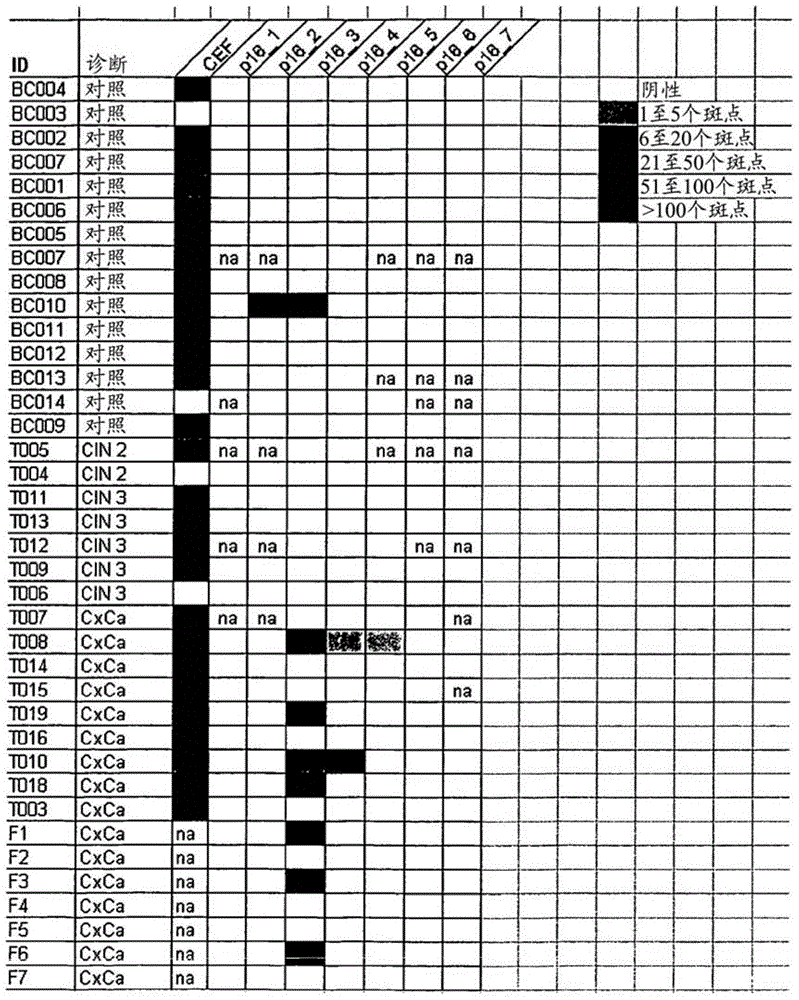
[0051] To evaluate whether patients with HPV-associated tumors increase strongly overexpressed p16 INK4a T cells against strongly overexpressed p16 INK4a To what extent T cells respond, the application allows detailed characterization of p16-targeted INK4a Different approaches to the immune response to antigens. Targeting p16 in cervical cancer patients INK4a The discovery of a spontaneous immune response demonstrates the immunogenicity of the general antigen as well as of the specific p16INK4a fragment and for immunization containing the p16INK4a fragment with expression of p16 INK4a patients with tumors provided rationale.
[0052] will come from 13 individuals with expression of p16 INK4a Peripheral blood mononuclear cells (PBMC) and p16 of women with high-grade cervical dysplasia (CIN2 / 3) INK4a Peptides (Table 1) were incubated with p16 by applying INK4a BrdU assay for ...
Embodiment 2
[0056] use p16 INK4a Peptides prime healthy donor T cells in vitro
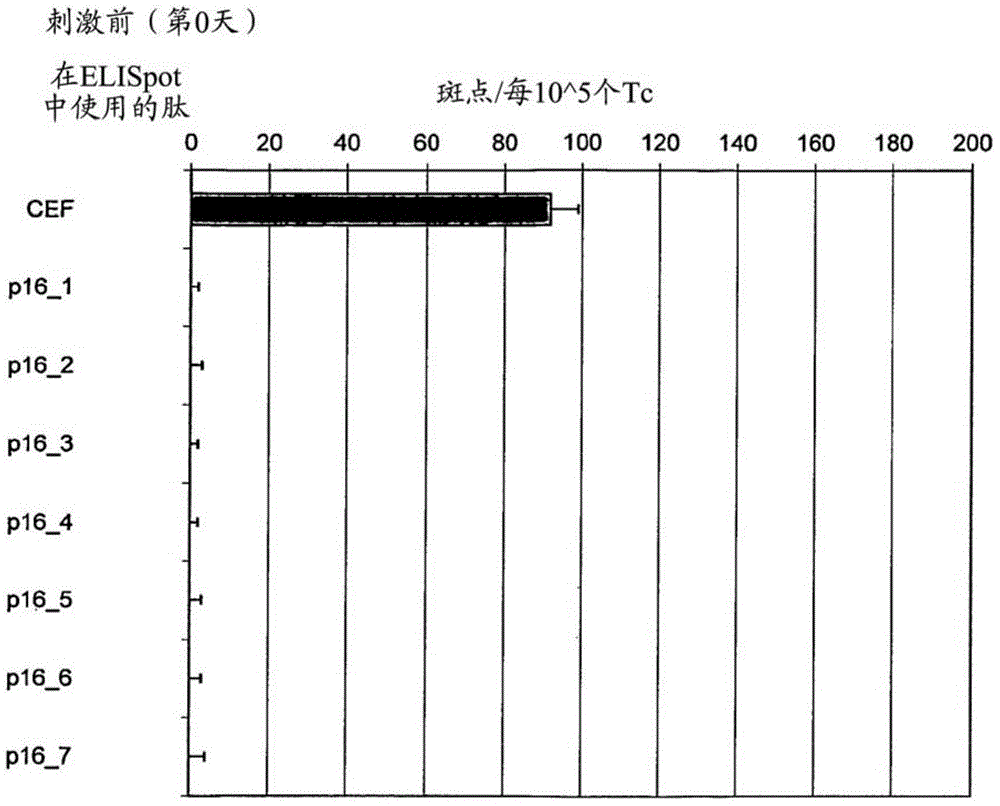
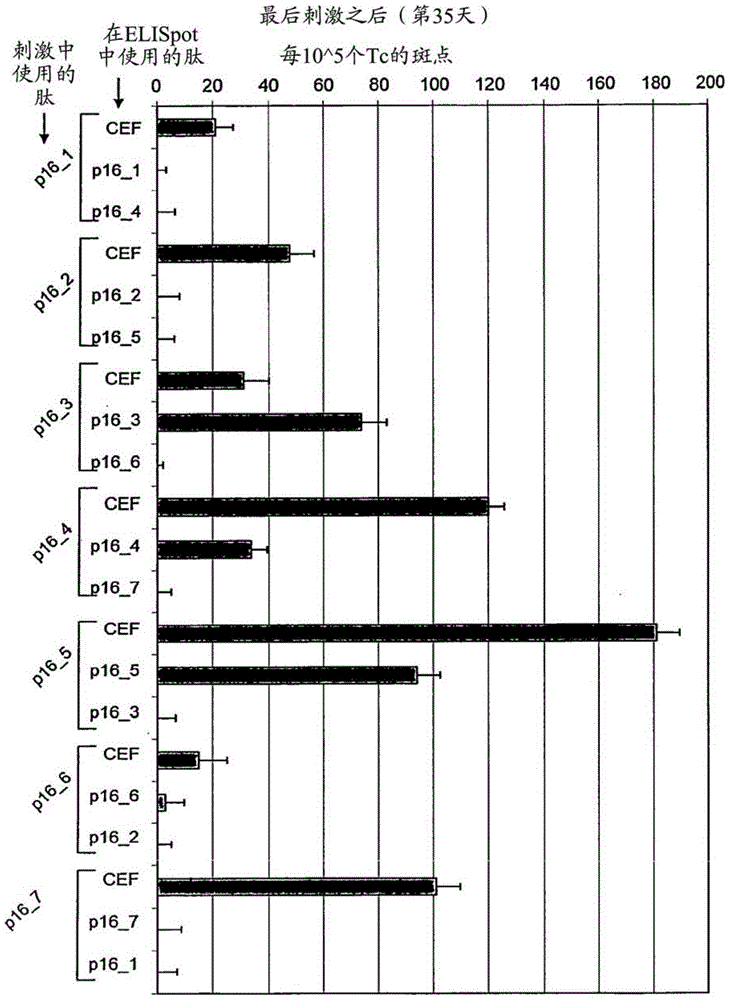
[0057] Tests covering the entire p16 INK4a Amino acid sequence of seven long 25-35mer peptides (each with 7-13 amino acid overlap) to define p16 capable of inducing T cells secreting interferon gamma in healthy donors in vitro INK4a Fragments (Table 1).
[0058] Table 1 Seven overlapping p16s used in in vitro experiments INK4a peptide
[0059]
[0060] To show p16 INK4a Fragment stimulates healthy donor T cells to secrete interferon gamma in vitro and identifies most immunogenic p16 INK4a source of epitopes, to investigate whether T cells isolated from the peripheral blood of healthy donors could be detected by these p16 INK4a Peptide stimulation in vitro. When using individual p16 in so-called ELISpot experiments INK4a During peptide challenge, if p16 INK4a Peptides are able to induce specific T cell responses in cell culture experiments, and T cells secrete cytokines. In the ELISpot assay, cyto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com