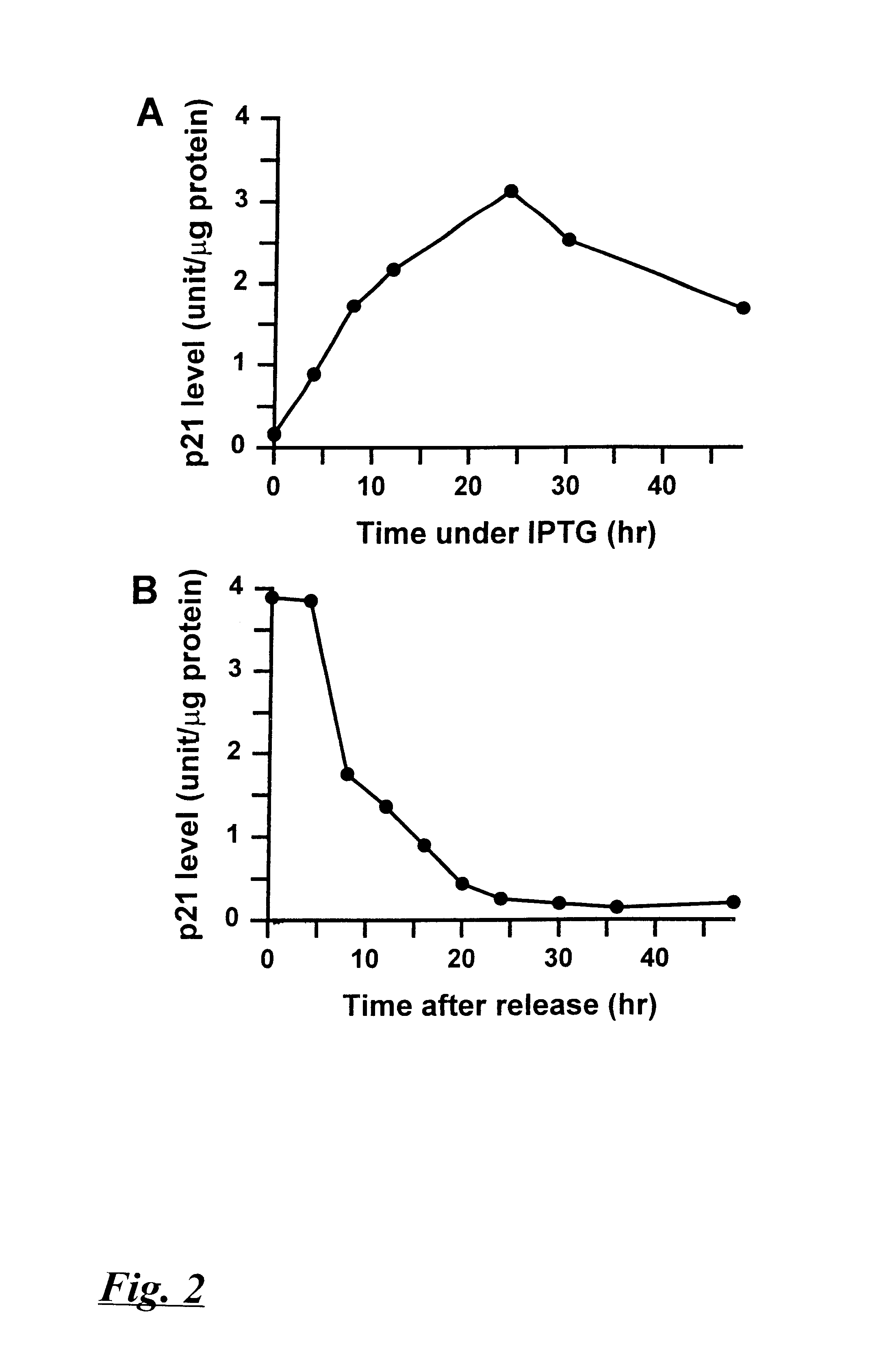
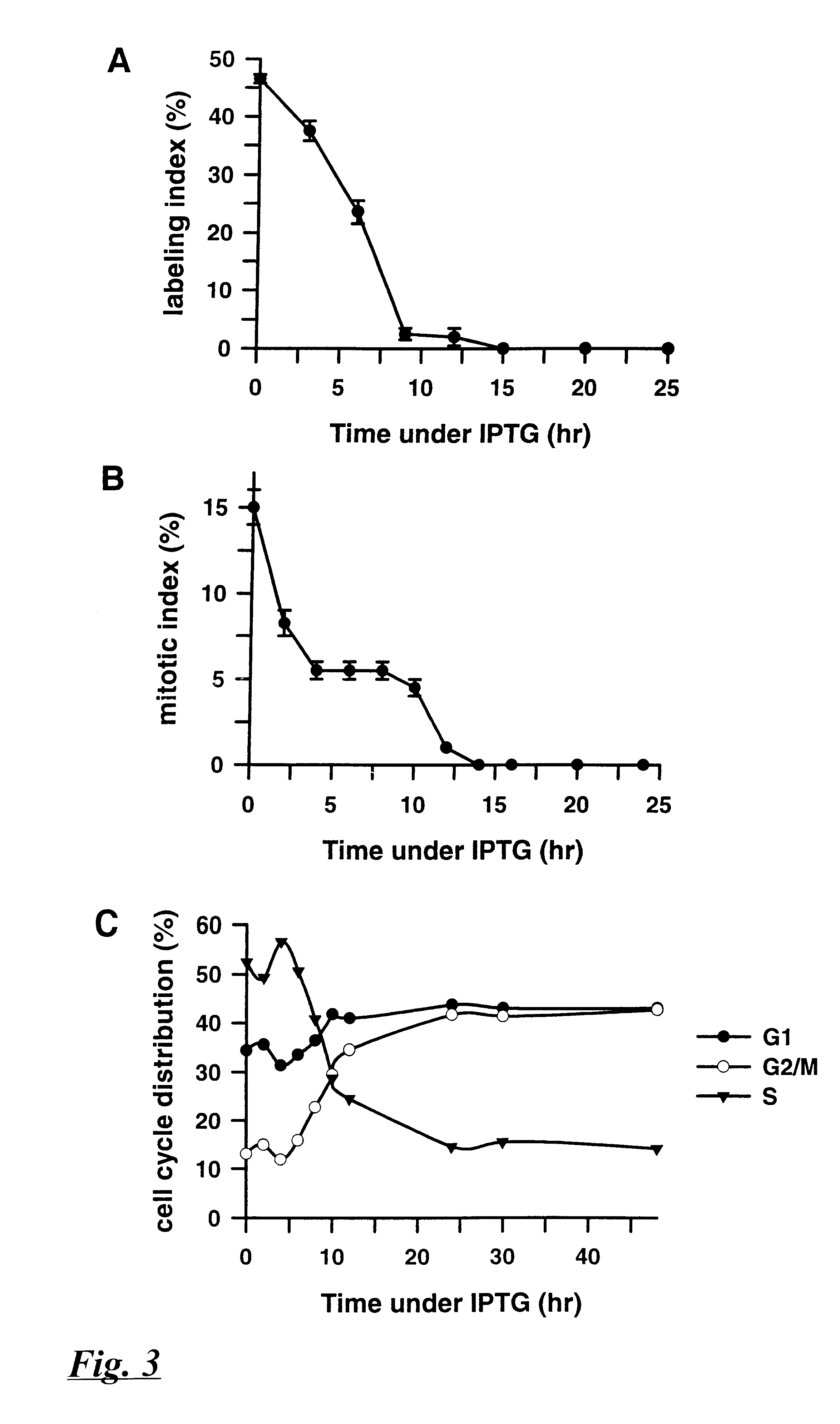
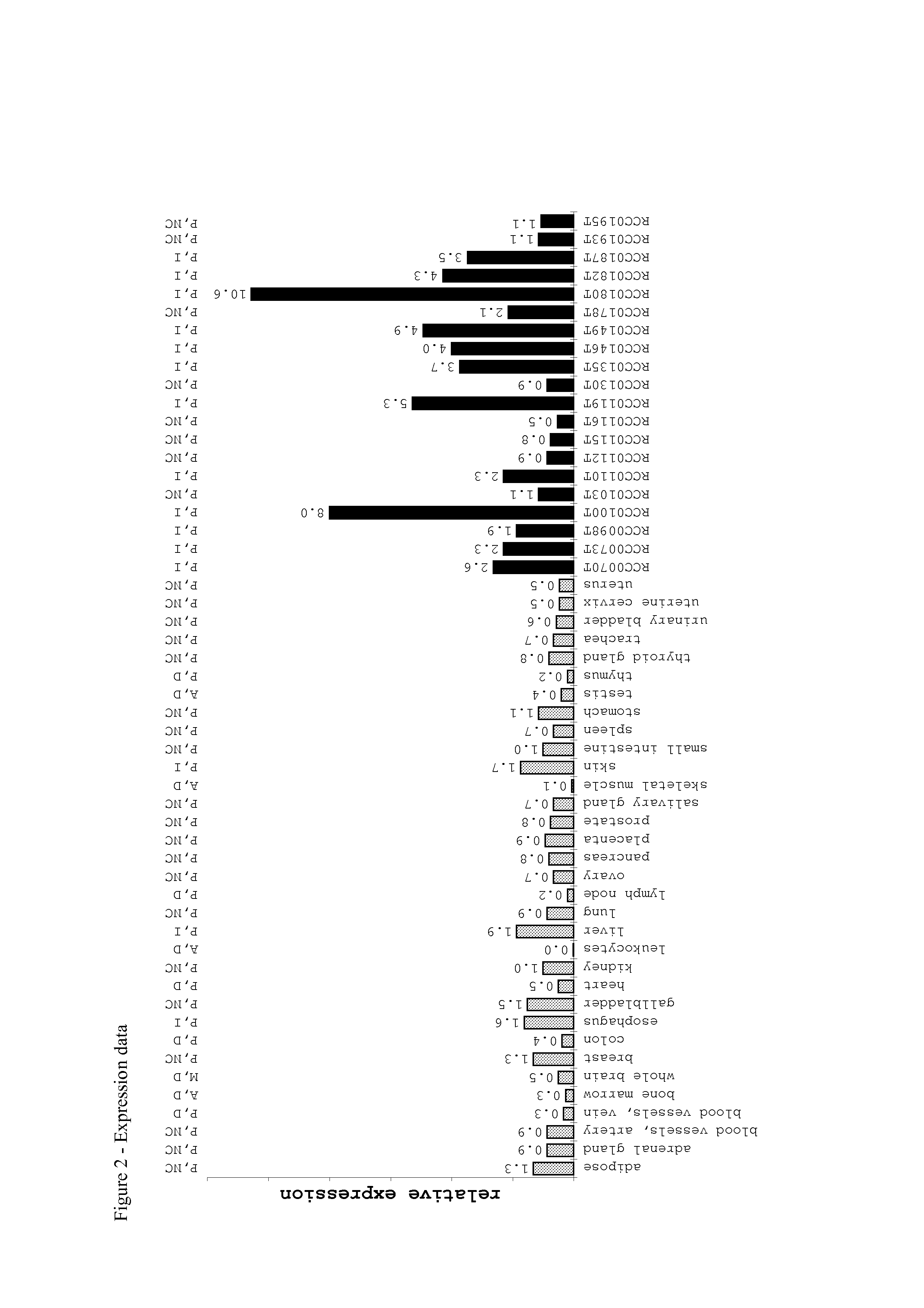
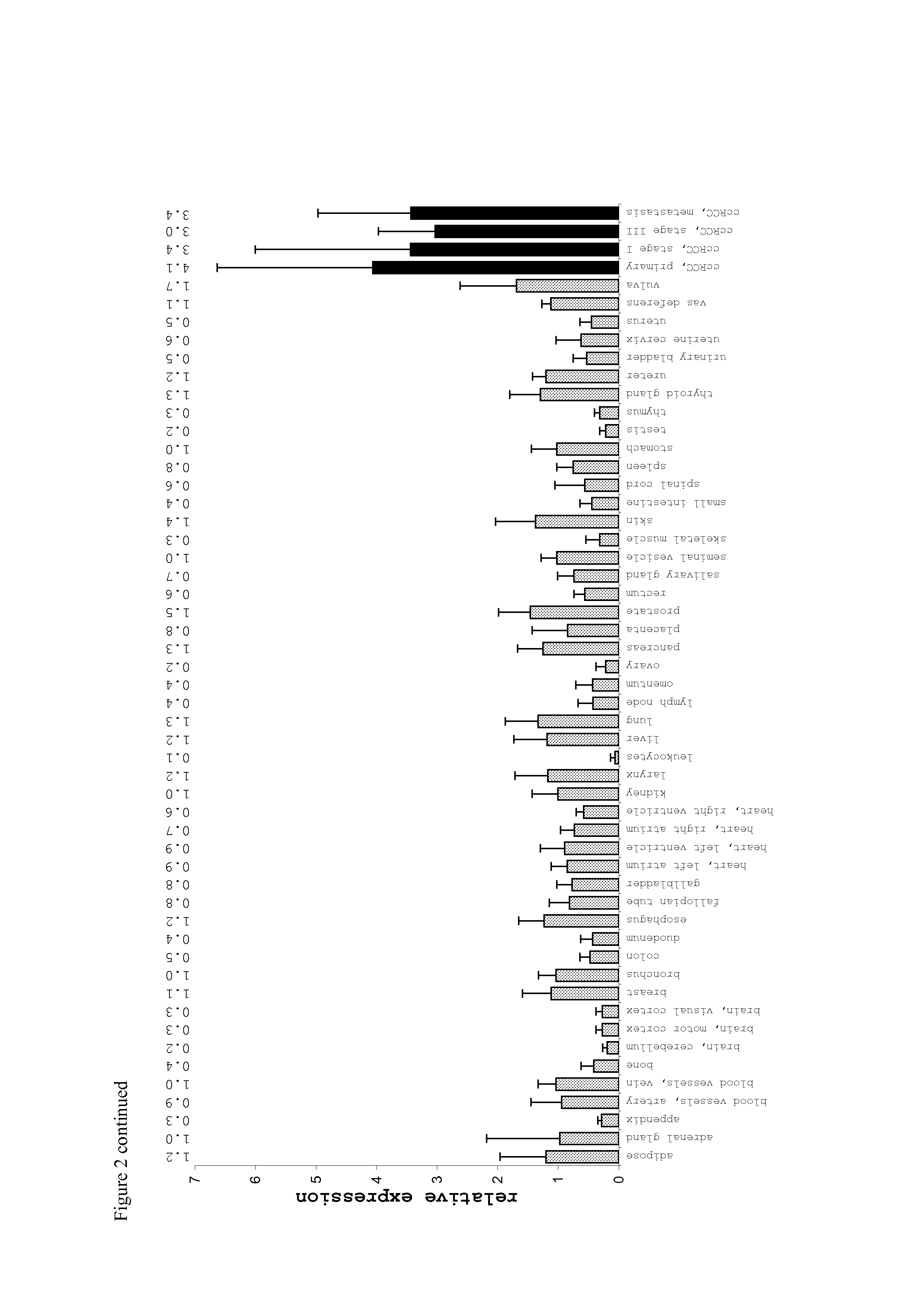
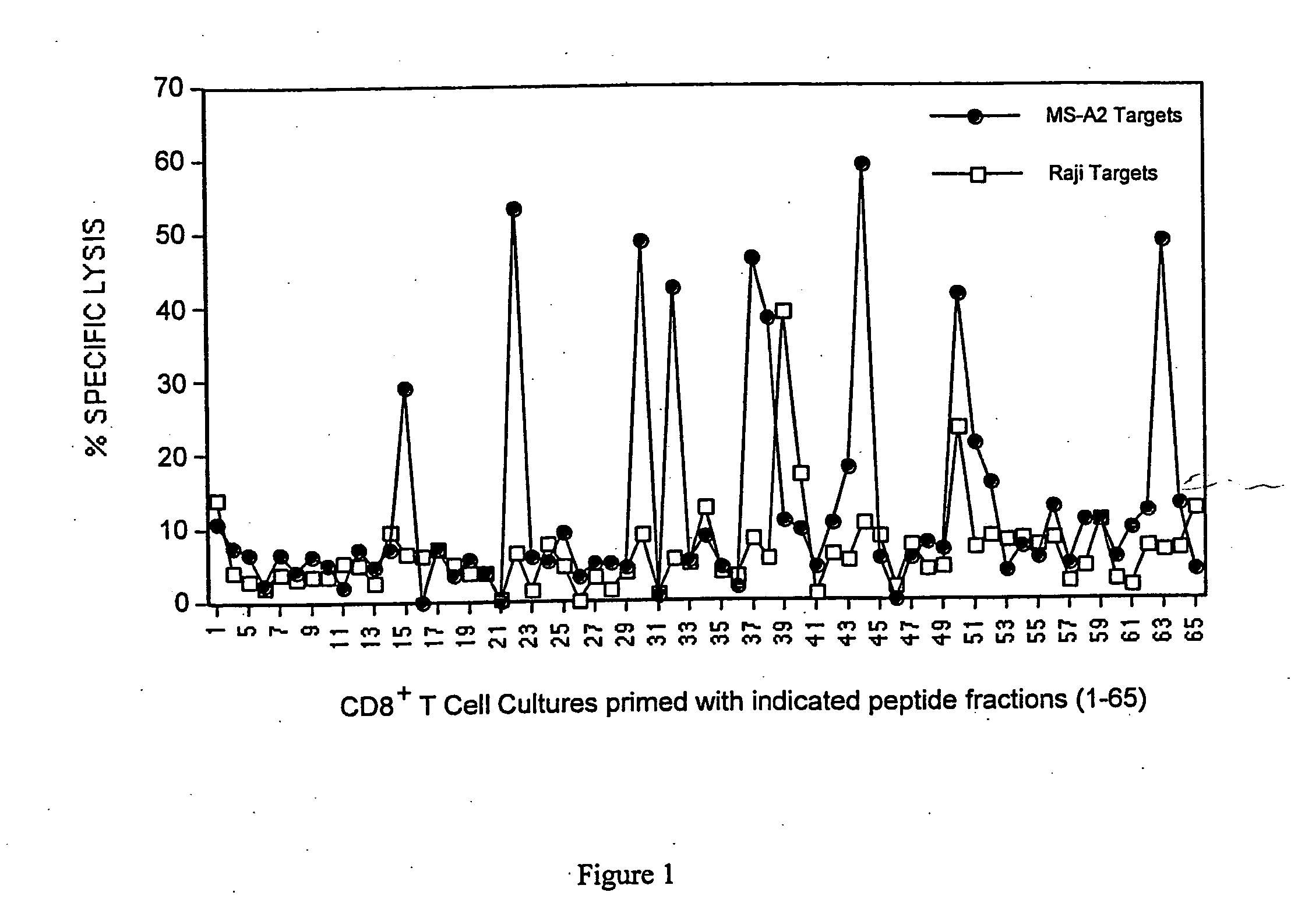
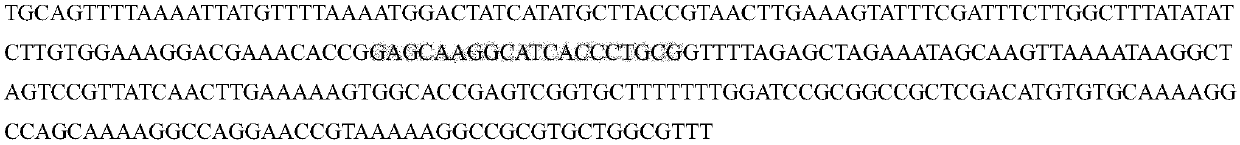Patents
Literature
154results about "Cell cycle regulated proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Conditionally immortalized long-term stem cells and methods of making and using such cells
ActiveUS20100297763A1Inhibit apoptosisPromotes proliferationVirus peptidesApoptosis related proteinsDiseaseStudy methods
Disclosed are methods for conditionally immortalizing stem cells, including adult and embryonic stem cells, the cells produced by such methods, therapeutic and laboratory or research methods of using such cells, and methods to identify compounds related to cell differentiation and development or to treat diseases, using such cells. A mouse model of acute myeloid leukemia (AML) and cells and methods related to such mouse model are also described.
Owner:NAT JEWISH HEALTH +1
Reagents and methods for identifying and modulating expression of genes regulated by p21
InactiveUS6706491B1Good curative effectInhibit cellular senescenceMicrobiological testing/measurementMicroorganism based processesGrowth promotionApoptosis
This invention provides methods and reagents for identifying genes involved in cell cycle progression, growth promotion, modulation of apoptosis, cellular senescence and aging, and methods for identifying compounds that inhibit or potentiate cellular senescence.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Cancer therapy based on tumor associated antigens derived from cyclin d1
The present invention relates to cyclin D1-derived peptides for use in the improved treatment of cancer in a patient, particularly in the form of a combination therapy using a vaccine. Other aspects relate to the use of the peptides or a combination thereof as a diagnostic tool.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
ARF-P19, a novel regulator of the mammalian cell cycle
InactiveUS6407062B1Peptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthCancer cell
The INK4A (MTS1, CDKN2) gene encodes a specific inhibitor (InK4a-p16) of the cyclin D-dependent kinases CDK4 and CDK6. InK4a-p16 can block these kinase from phosphorylating the retinoblastoma protein (pRb), preventing exit from the G1 phase of the cell cycle. Deletions and mutations involving the gene encoding InK4a-p16, INK4A, occur frequently in cancer cells, implying that INK4a-p16, like pRb, suppresses tumor formulation. However, a completely unrelated protein (ARF-p19) arises in major part from an alternative reading frame of the mouse INK4A gene. Expression of an ARF-p19 cDNA (SEQ ID NO:1) in rodent fibroblasts induces both G1 and G2 phase arrest. Economical reutilization of protein coding sequences in this manner is without precedent in mammalian genomes, and the unitary inheritance of INK4a-p16 and ARF-p19 may reflect a dual requirement for both proteins in cell cycle control.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Cell regulatory genes, encoded products, and uses related thereto
InactiveUS6946256B1Peptide/protein ingredientsImmunoglobulins against animals/humansSuppressorApoptosis
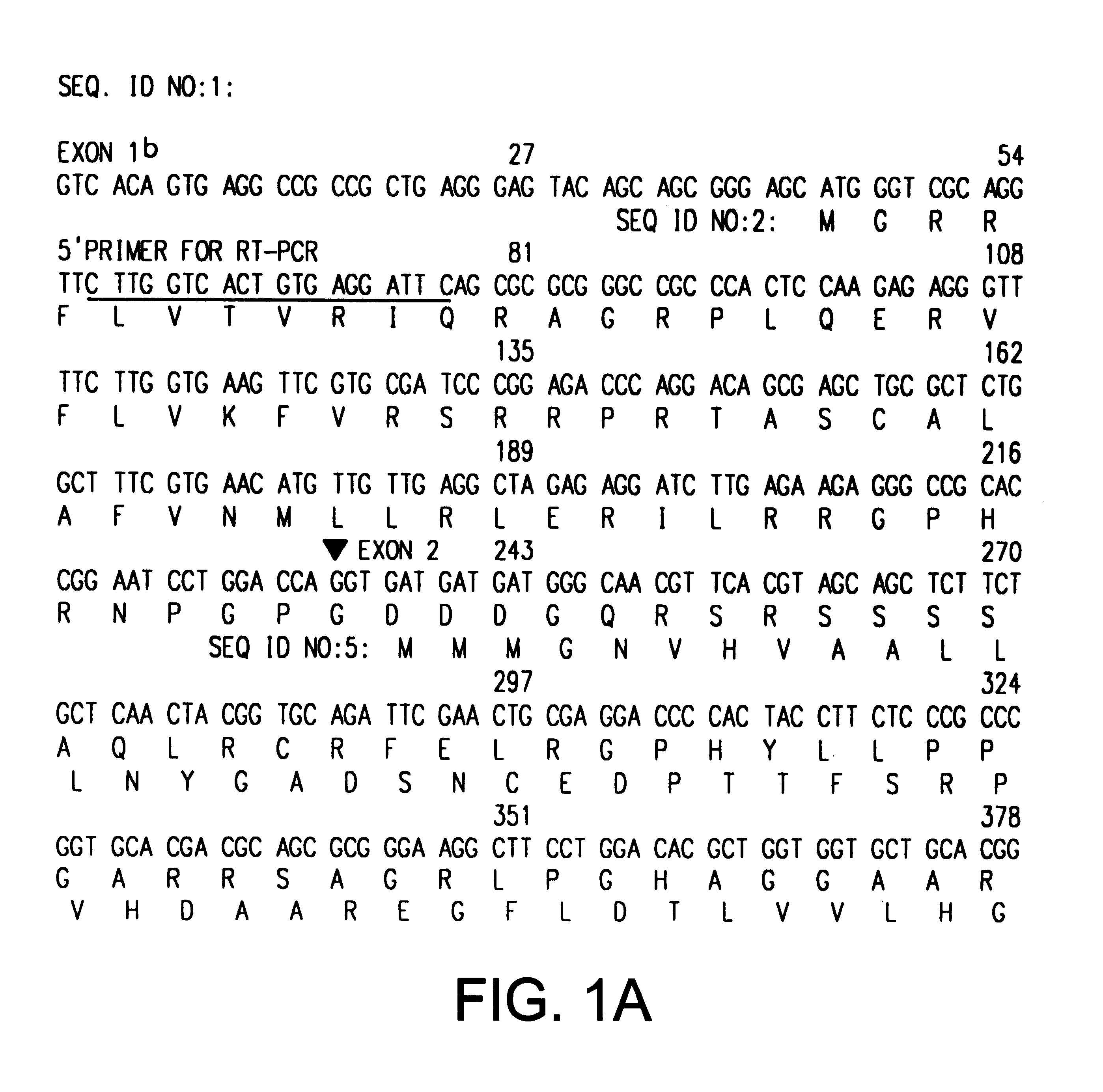
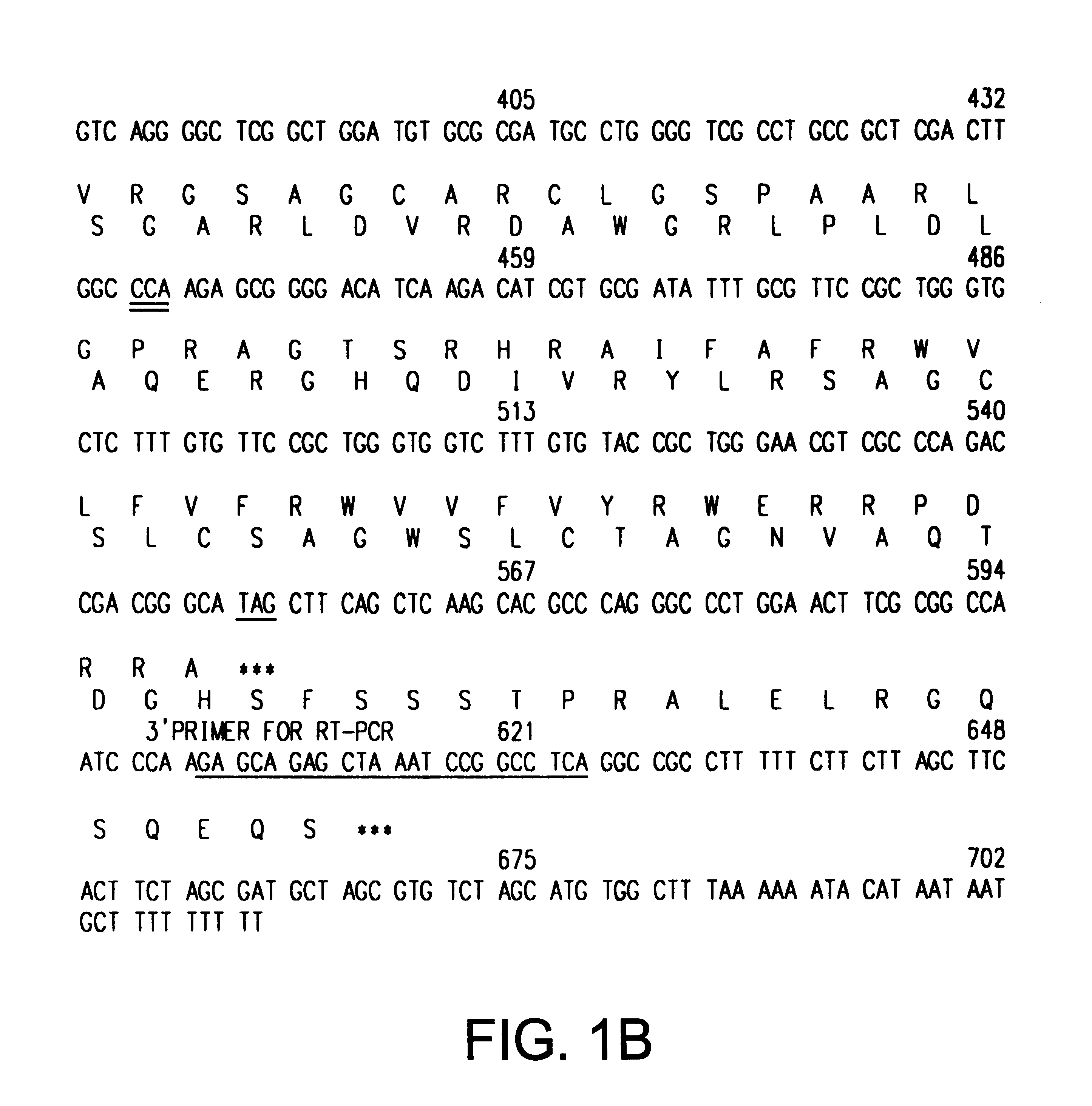
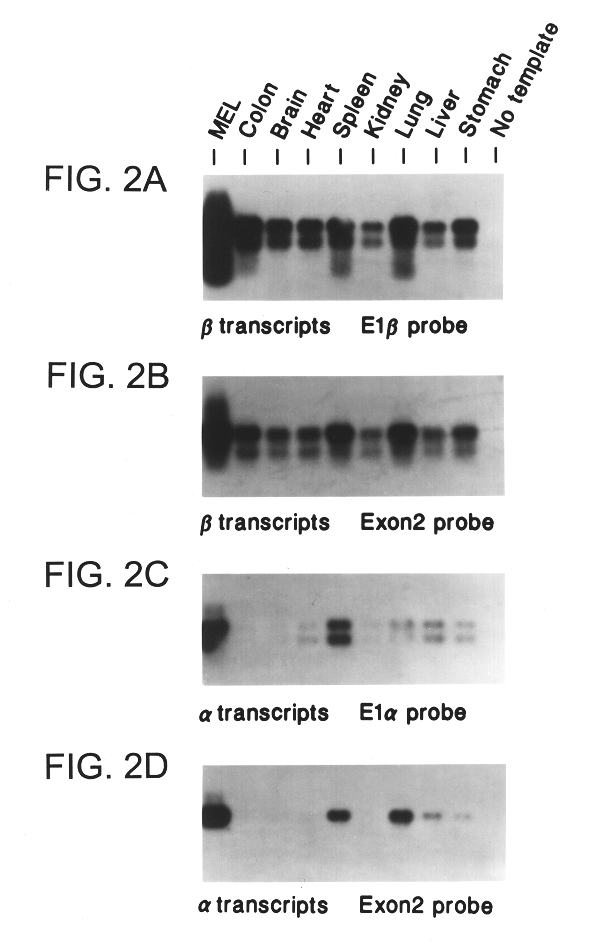
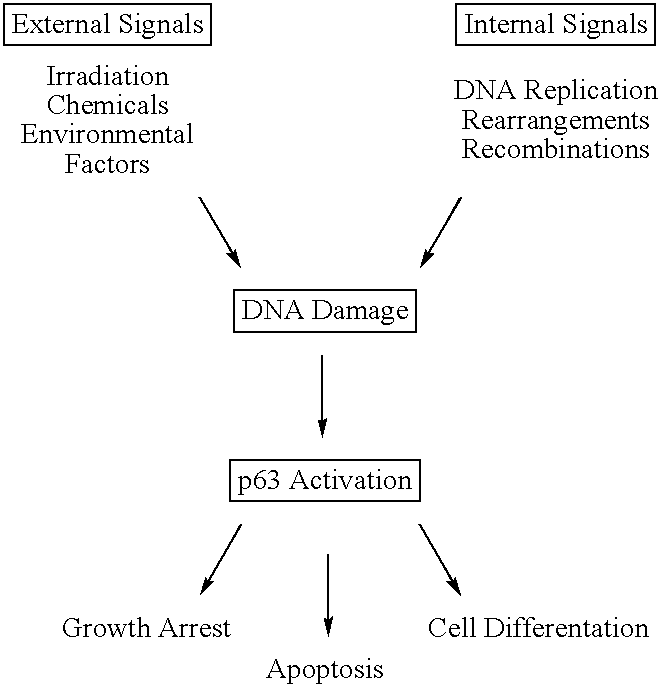
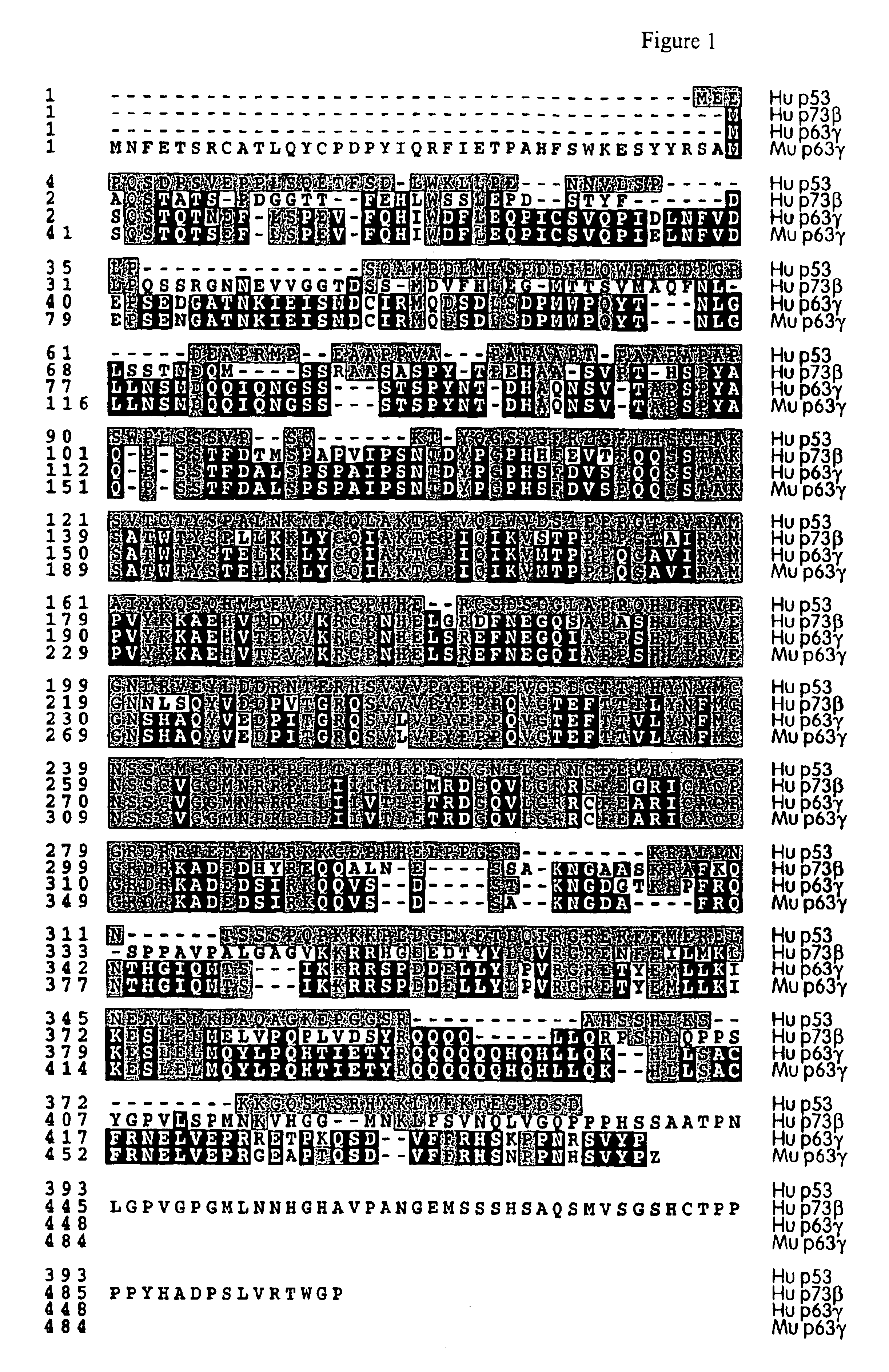
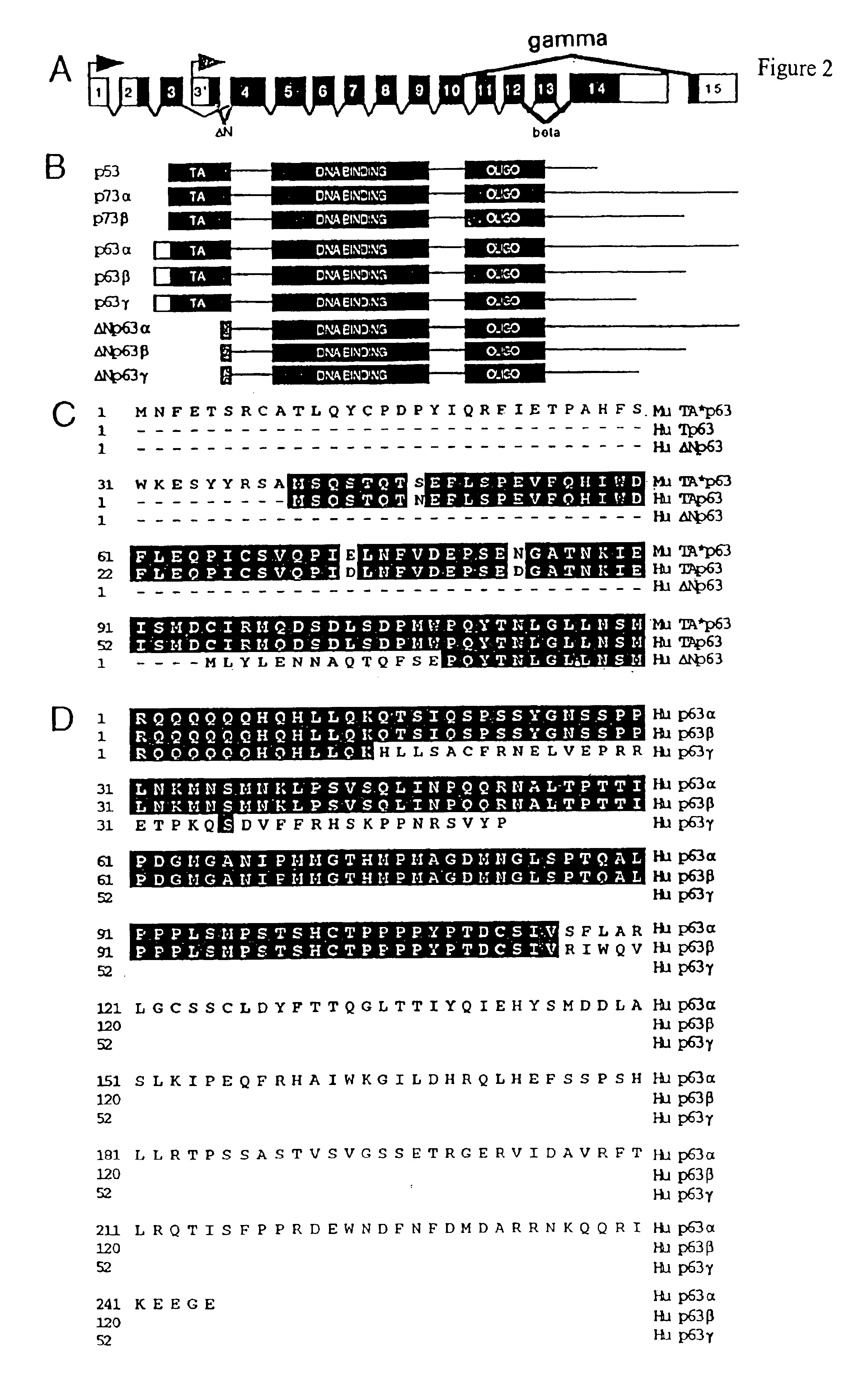
This application describes the cloning of p63, a gene at chromosome 3q27-29, that bears homology to the tumor suppressor p53. The p63 gene encodes at least six different isotypes. p63 was detected in a variety of human and mouse tissue and demonstrates remarkably divergent activities, such as the ability to transactivate p53 reporter genes and induce apoptosis. Isotopes of p63 lacking a transactivation domain act as dominant negatives towards the transactivation by p53 and p63.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +2
Cancer therapy based on tumor associated antigens derived from cyclin d1
The present invention relates to cyclin D1-derived peptides for use in the improved treatment of cancer in a patient, particularly in the form of a combination therapy using a vaccine. Other aspects relate to the use of the peptides or a combination thereof as a diagnostic tool.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
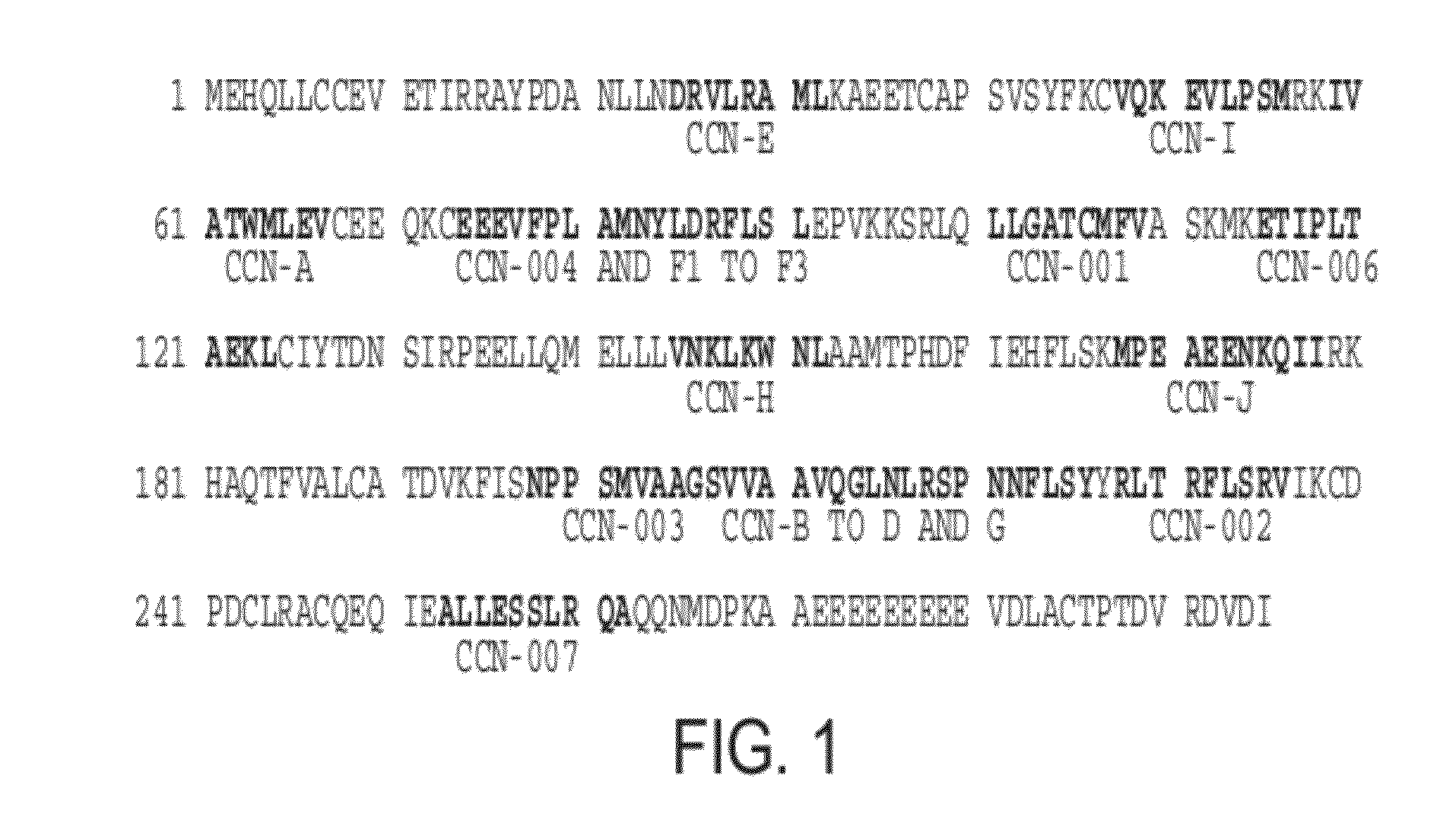
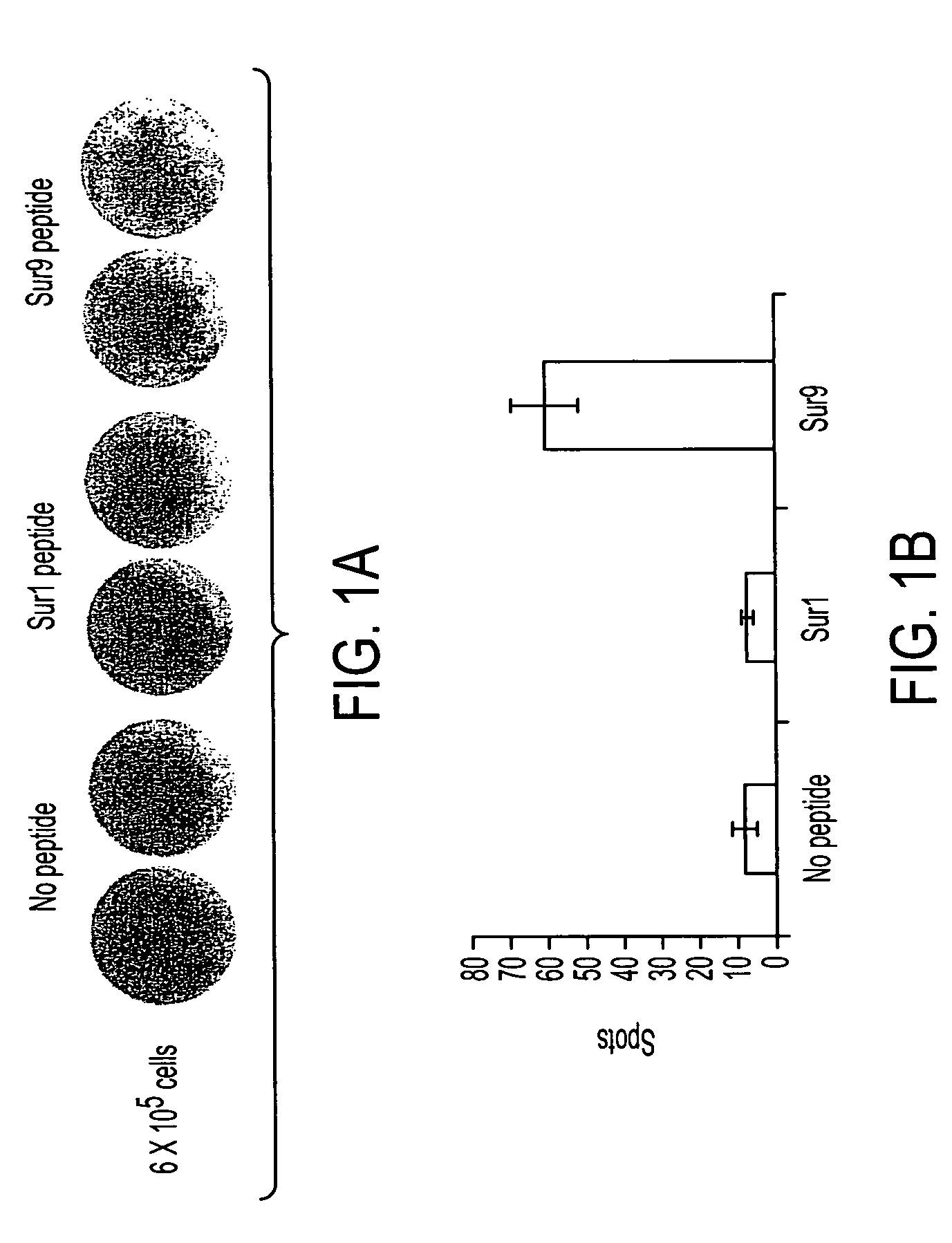
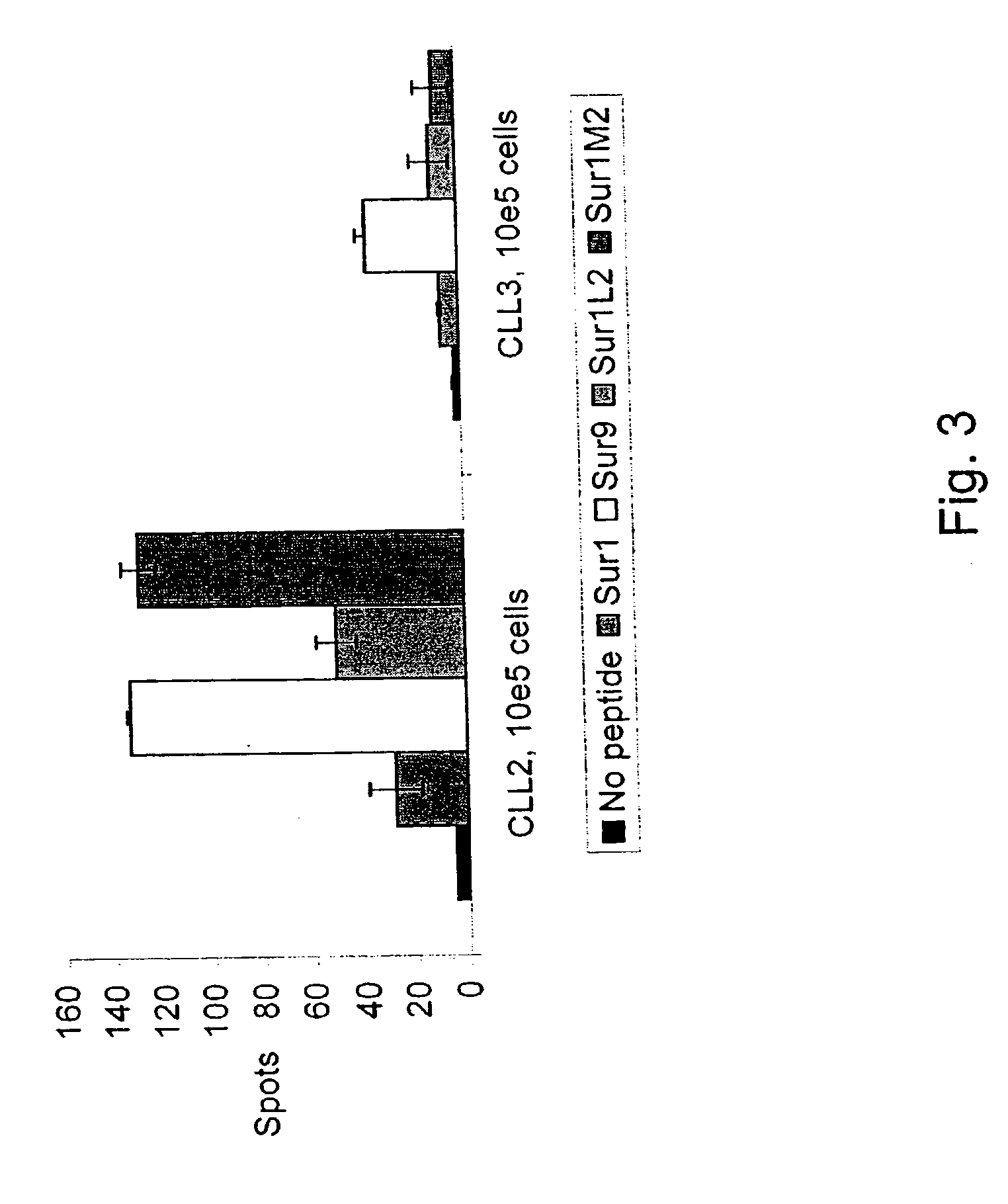
Survivin-derived peptides and use thereof
InactiveUS20040210035A1Easy to filterWeak affinityPeptide/protein ingredientsDepsipeptidesMHC class IWilms' tumor
MHC Class I-restricted peptides derived from the tumor associated antigen, survivin, which peptides are capable of binding to Class I HLA molecules at a high affinity, capable of eliciting INF-gamma-producing cells in a PBL population of a cancer patient and capable of in situ detection of cytotoxic T cells in a tumor tissue, therapeutic and diagnostic composition comprising the peptide and uses hereof.
Owner:SURVAC
Methods And Marker Combinations For Screening For Predisposition To Lung Cancer
InactiveUS20120071334A1Peptide/protein ingredientsLibrary screeningLung cancer susceptibilityBiomarker (petroleum)
The present invention relates to rapid, sensitive methods for determining whether a subject has or is at risk of developing lung cancer based on certain combinations of biomarkers, or biomarkers and biometric parameters. The methods consist of: a) quantifying in a test sample obtained from a subject, the amount of two or more biomarkers in a panel, the panel comprising at least one antibody and at least one antigen: b) comparing the amount of each biomarker quantified in the panel to a predetermined cutoff for said biomarker and assigning a score for each biomarker based on said comparison: c) combining the assigned score for each biomarker quantified in step b to obtain a total score for said subject: d) comparing the total score in step c with a predetermined total score and e) determining whether said subject has a risk of lung cancer based on the comparison in step d.
Owner:ABBOTT MOLECULAR INC
Cell cycle control protein
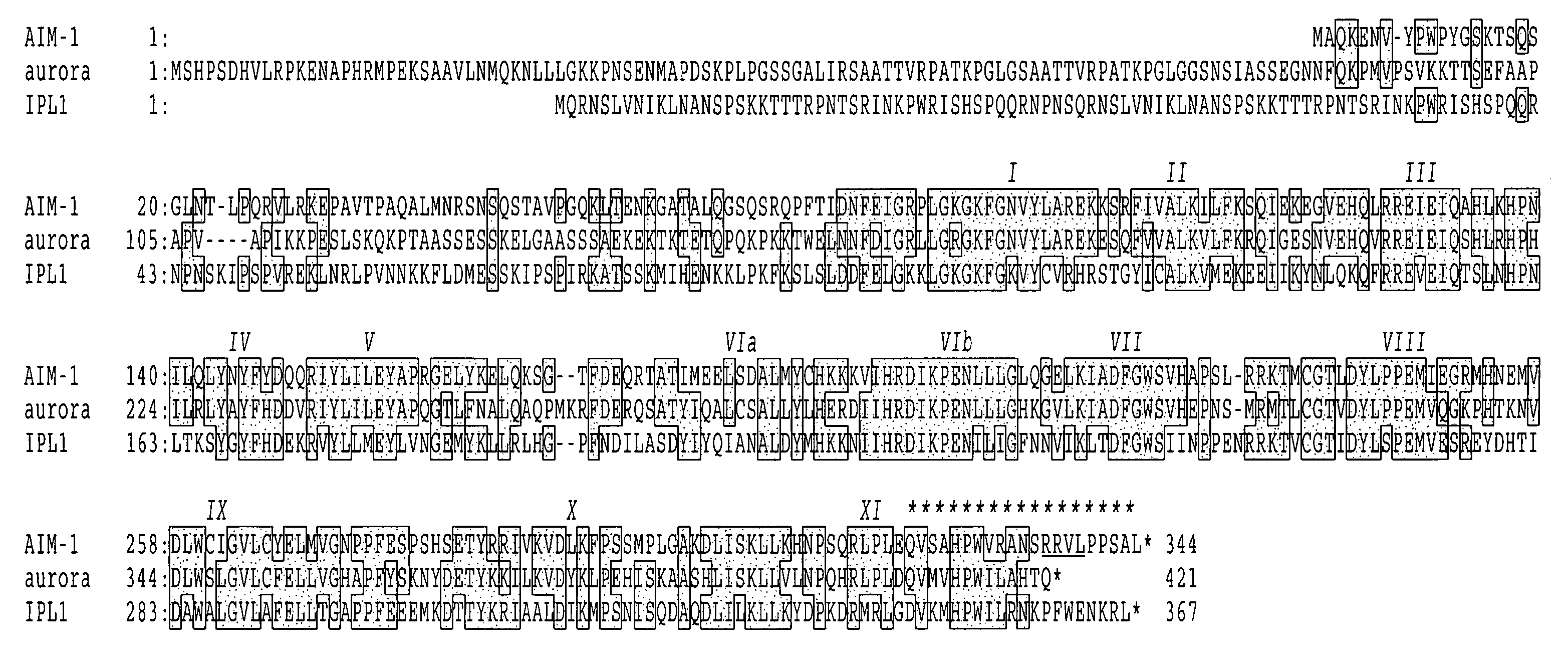
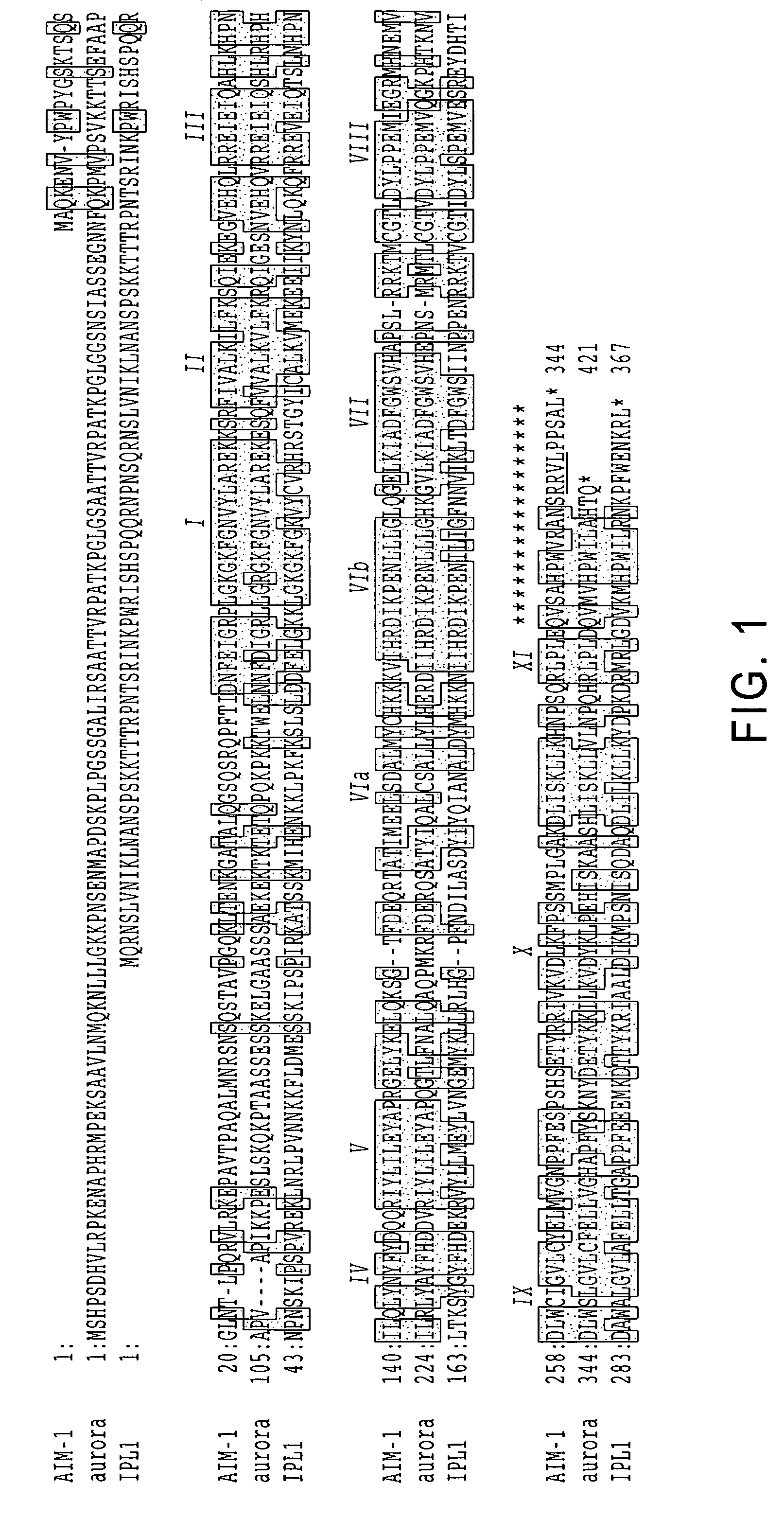
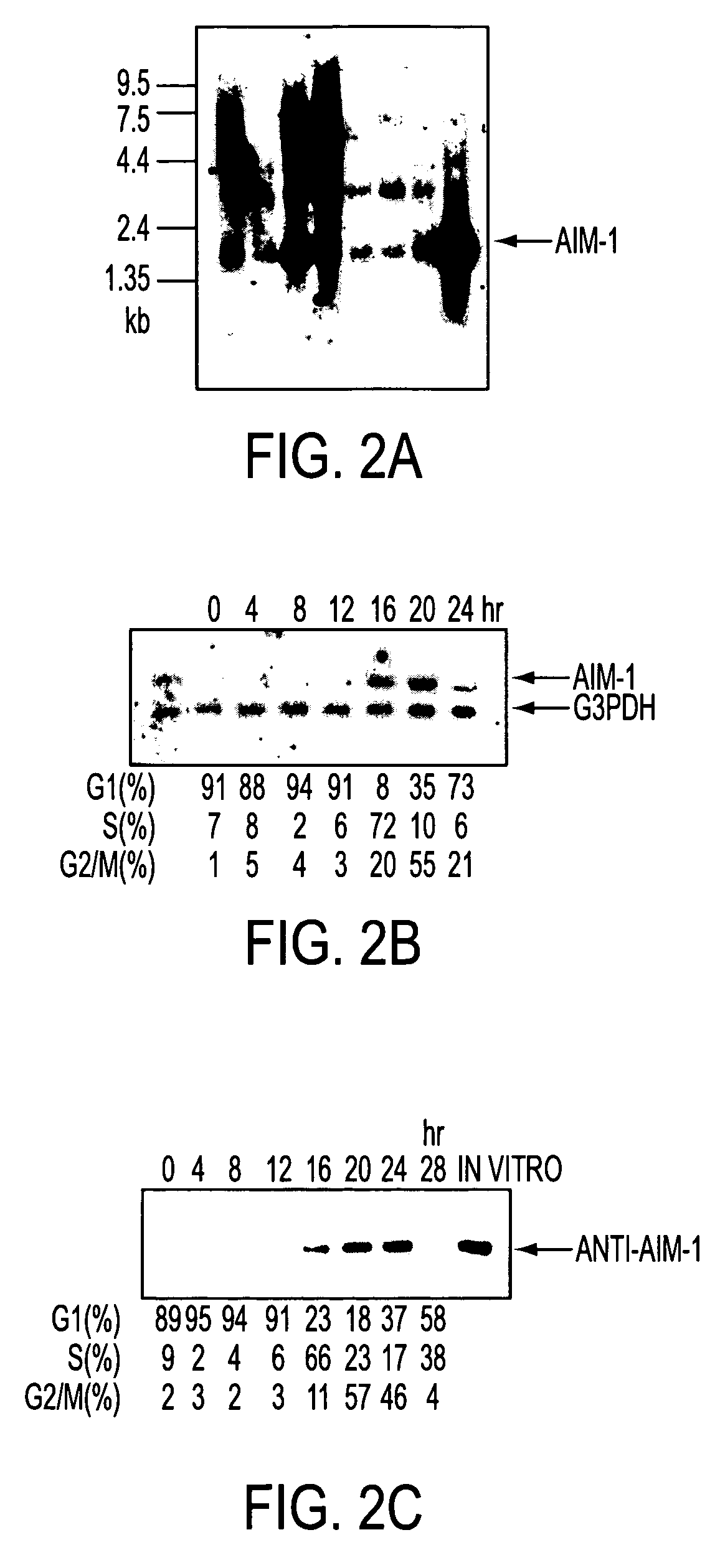
A DNA containing a nucleotide sequence encoding the amino acid sequence shown as SEQ ID NO: 2 in Sequence Listing, or a nucleotide sequence encoding a protein having the amino acid sequence shown as SEQ ID NO: 2 in Sequence Listing with partial substitution, deletion or addition and having cell cycle control activity, or a nucleotide sequence hybridizing to them; a recombinant vector containing said DNA; a cell transformed with said vector; a process for producing AIM-1 using said DNA; a recombinant AIM-1 protein obtained by said process; an oligonucleotide or peptide nucleic acid capable of specifically hybridizing to a nucleotide sequence encoding AIM-1 protein; an antibody recognizing AIM-1; a therapeutic agent for diseases associated with abnormal cell growth containing an inhibitor against AIM-1 protein; and a screening method for materials having serine-threonine inhibitory activity using AIM-1 gene or AIM-1 protein.
Owner:HIROSHIMA UNIVERSITY
Animal models of pancreatic adenocarcinoma and uses therefor
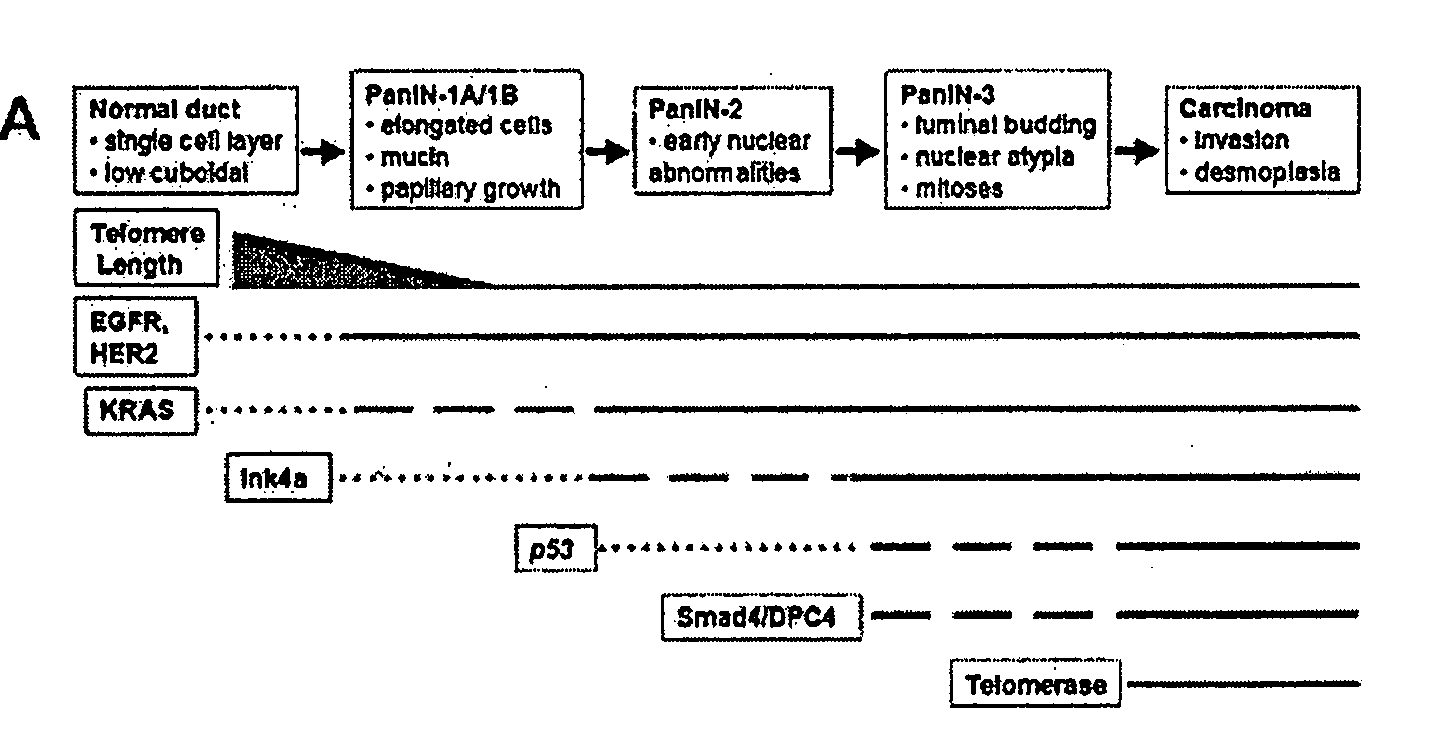
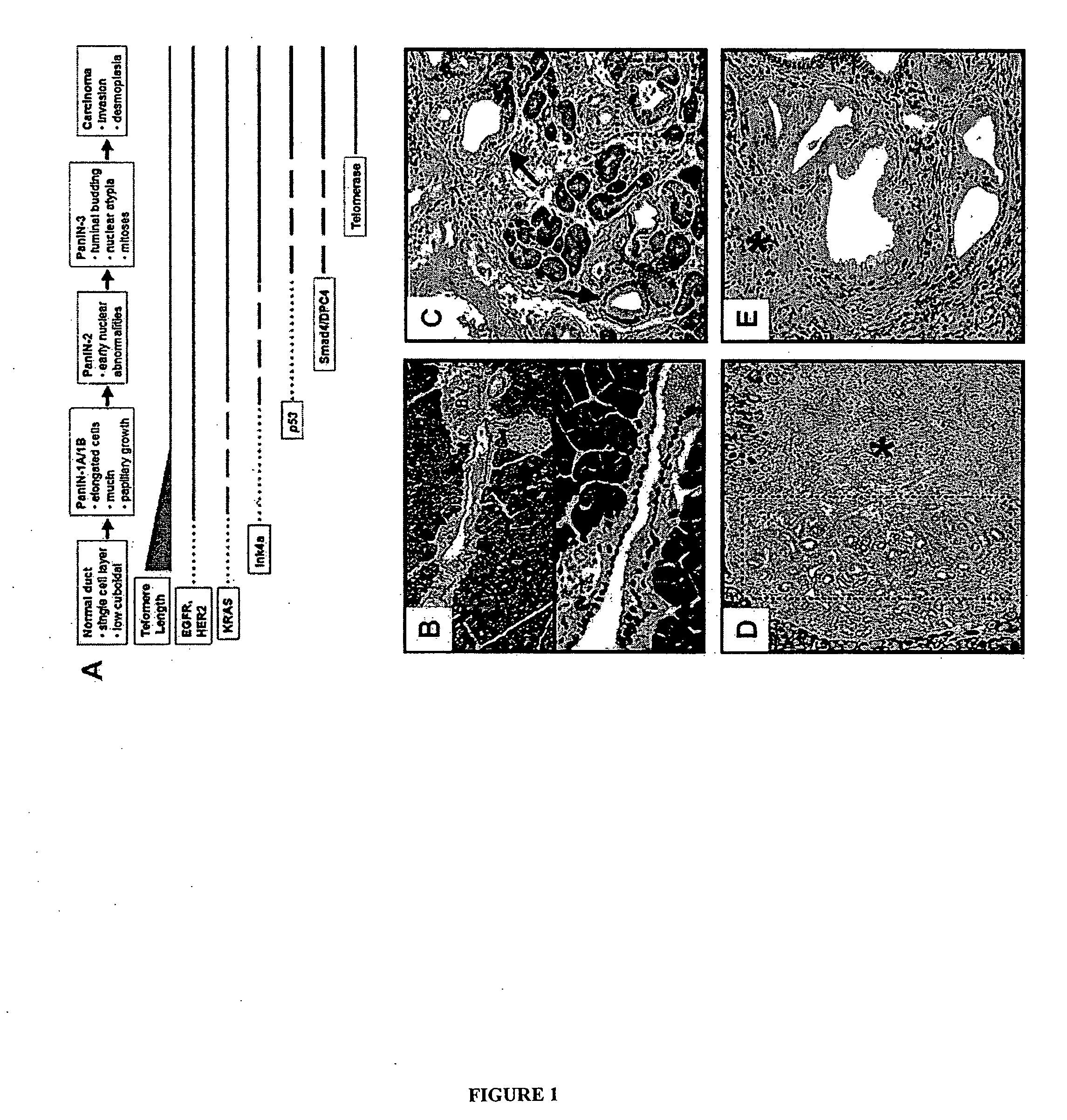
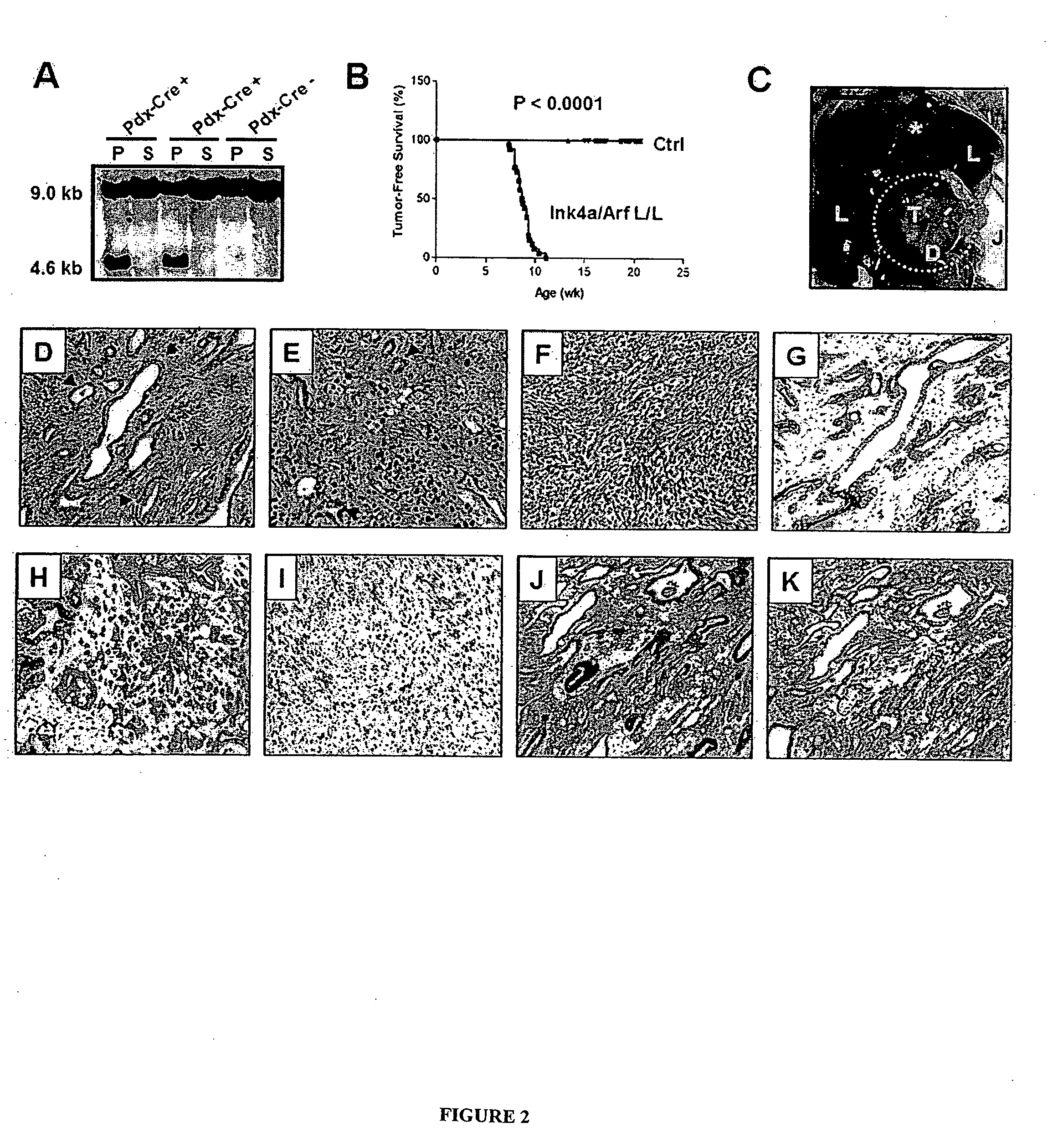
The present invention is based, at least in part, on the generation of an animal model of pancreatic adenocarcinoma which recapitulates the genetic and histological features of human pancreatic adenocarcinoma, including the initiation, maintenance, and progression of the disease. Accordingly, the present invention provides animal models of cancer, e.g., pancreatic adenocarcinoma, wherein an activating mutation of Kras has been introduced, and any one or more known or unknown tumor suppressor genes or loci, e.g., Ink4a / Arf, Ink4a, Arf, p53, Smad4 / Dpc, Lkb1, Brca2, or Mlh1, have been misexpressed, e.g., have been misexpressed leading to decreased expression or non-expression. The animal models of the invention may be used, for example, to identify biomarkers of pancreatic cancer, to identify agents for the treatment or prevention of pancreatic cancer, and to evaluate the effectiveness of potential therapeutic agents.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Yeast mammalian regulators of cell proliferation
InactiveUS6383753B1Quick identificationQuick functionSugar derivativesMicrobiological testing/measurementYeastAntagonism
Owner:RGT UNIV OF MICHIGAN
Human diaphanous-3 gene and methods of use therefor
InactiveUS20050054826A1Cell receptors/surface-antigens/surface-determinantsSugar derivativesFull length cdnaMitotic cell
The present invention is directed to the full-length cDNA sequence encoding human diaphanous-3 (DIAPH3), to DIAPH3 encoded thereby, and to fragments of DIAPH3 and the cDNA. The present invention also provides for the use of the cDNA, and of DIAPH3, as a marker of poor prognosis of breast cancer. Because DIAPH3 appears essential for proper spindle pole formation during mitosis, DIAPH3 is a useful target for screening assays designed to identify inhibitors or modulators of DIAPH3 activity, which are useful for the treatment of cancer, particularly breast cancer. Thus, the invention further provides methods of using DIAPH3, or fragments thereof, in assays to identify such compounds.
Owner:ROSETTA INPHARMATICS LLC
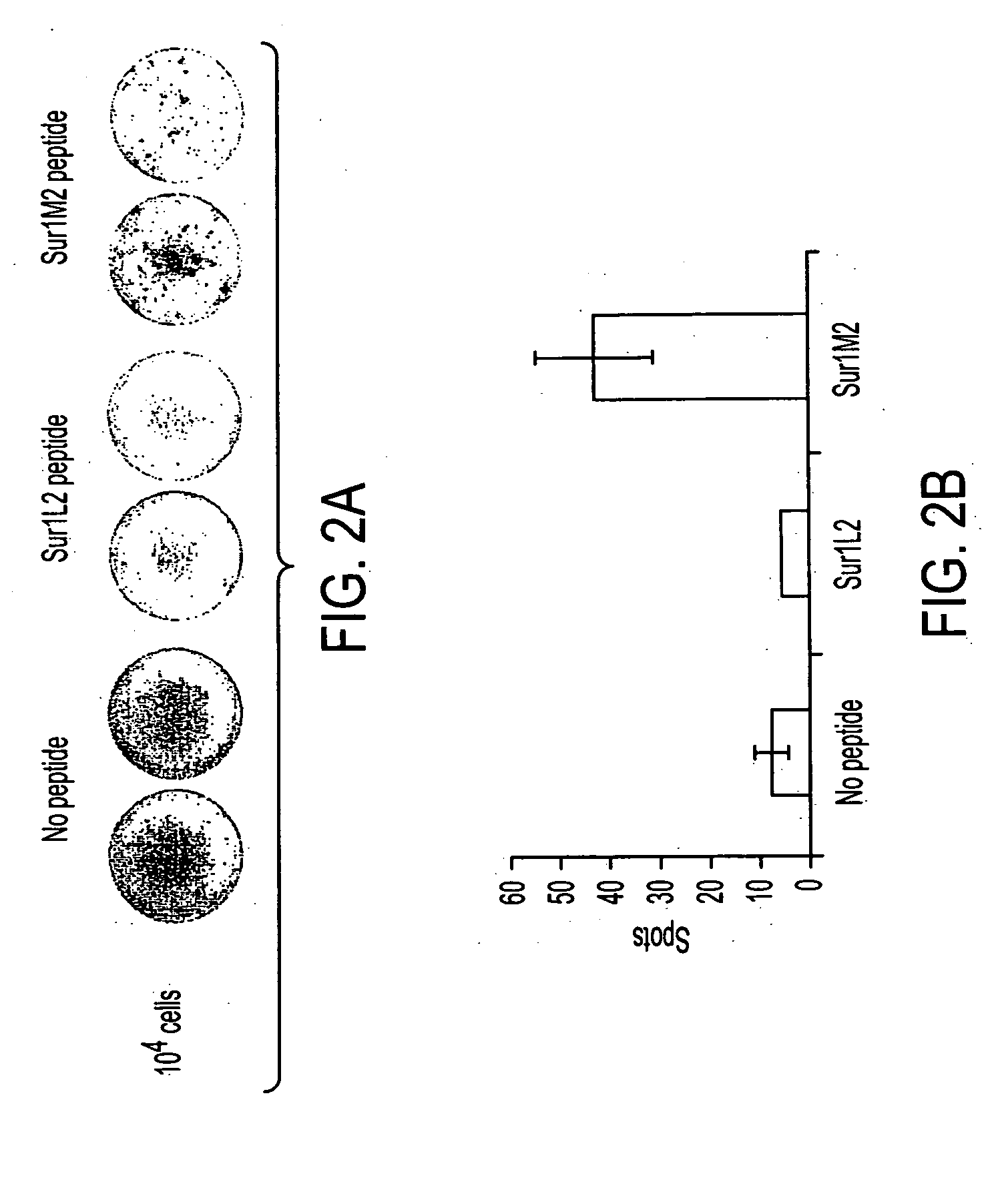
Survivin-derived peptides and use thereof
ActiveUS20040176573A1Easy to filterIncrease the immuogenicity of survivin-derived peptidesPeptide/protein ingredientsApoptosis related proteinsMHC class IWilms' tumor
MHC Class I-restricted peptides derived from the tumor associated antigen, survivin, which peptides are capable of binding to Class I HLA molecules at a high affinity, capable of eliciting INF-gamma-producing cells in a PBL population of a cancer patient and capable of in situ detection of cytotoxic T cells in a tumor tissue, therapeutic and diagnostic composition comprising the peptide and uses hereof.
Owner:SURVAC
Monoclonal antibodies and methods for their use in the detection of cervical disease
InactiveUS20070117167A1Animal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseEpitope
Owner:TRIPATH IMAGING INC
MCM6 and MCM7 monoclonal antibodies and methods for their use in the detection of cervical disease
Compositions and methods for diagnosing high-grade cervical disease in a patient sample are provided. The compositions include novel monoclonal antibodies, and variants and fragments thereof, that specifically bind to MCM6 or MCM7. Monoclonal antibodies having the binding characteristics of an MCM6 or MCM7 antibody of the invention are further provided. Hybridoma cell lines that produce an MCM6 or MCM7 monoclonal antibody of the invention are also disclosed herein. The compositions find use in practicing methods for diagnosing high-grade cervical disease comprising detecting overexpression of MCM6, MCM7, or both MCM6 and MCM7 in a cervical sample from a patient. Kits for practicing the methods of the invention are further provided. Polypeptides comprising the amino acid sequence for an MCM6 or an MCM7 epitope and methods of using these polypeptides in the production of antibodies are also encompassed by the present invention.
Owner:TRIPATH IMAGING INC
Anticancer vaccine and diagnostic methods and reagents
The invention provides a method for vaccinating a patient against a malignancy comprising introducing a protein or peptide comprising of all or an immunogenic fragment of a cyclin protein into the patient. The invention further provides a method of identifying tumor antigens.
Owner:UNIVERSITY OF PITTSBURGH +1
Mammalian checkpoint genes and proteins
InactiveUS6307015B1Increased phosphorylationPeptide/protein ingredientsPeptide preparation methodsAbnormal tissue growthMammal
The present invention relates to the isolation of gene sequences encoding mammalian cell cycle checkpoints, as well as the expression of the encoded proteins using recombinant DNA technology. The expressed proteins are used to generate specific antibodies and to inhibit the growth of cells. The human checkpoint gene sequences are used as a probe for a portion of the chromosome associated with tumors and other malignancies, as well as growth and / or development deficiencies.
Owner:BAYLOR COLLEGE OF MEDICINE
Cytotoxic T-lymphocyte-inducing immunogens for prevention, treatment, and diagnosis of cancer
InactiveUS7083789B2Readily inducesHigh affinityTumor rejection antigen precursorsPeptide/protein ingredientsT lymphocytePolynucleotide
The present invention relates to compositions and methods for the prevention, treatment, and diagnosis of cancer, especially carcinomas, such as ovarian carcinoma. The invention discloses peptides, polypeptides, and polynucleotides that can be used to stimulate a CTL response against cancer.
Owner:ARGONEX PHARMA +1
ARF-P19, a novel regulator of the mammalian cell cycle
The INK4A (MTS1, CDKN2) gene encodes a specific inhibitor (InK4a-p16) of the cyclin D-dependent kinases CDK4 and CDK6. InK4a-p16 can block these kinase from phosphorylating the retinoblastoma protein (pRb), preventing exit from the G1 phase of the cell cycle. Deletions and mutations involving the gene encoding InK4a-p16, INK4A, occur frequently in cancer cells, implying that INK4a-p16, like pRb, suppresses tumor formulation. However, a completely unrelated protein (ARF-p19) arises in major part from an alternative reading frame of the mouse INK4A gene. Expression of an ARF-p19 cDNA (SEQ ID NO:1) in rodent fibroblasts induces both G1 and G2 phase arrest. Economical reutilization of protein coding sequences in this manner is without precedent in mammalian genomes, and the unitary inheritance of INK4a-p16 and ARF-p19 may reflect a dual requirement for both proteins in cell cycle control.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Cell-cycle checkpoint genes
InactiveUS20060205020A1Enhance specific toxicityImprove efficiencyPeptide/protein ingredientsTissue cultureFhit geneSchizosaccharomyces pombe
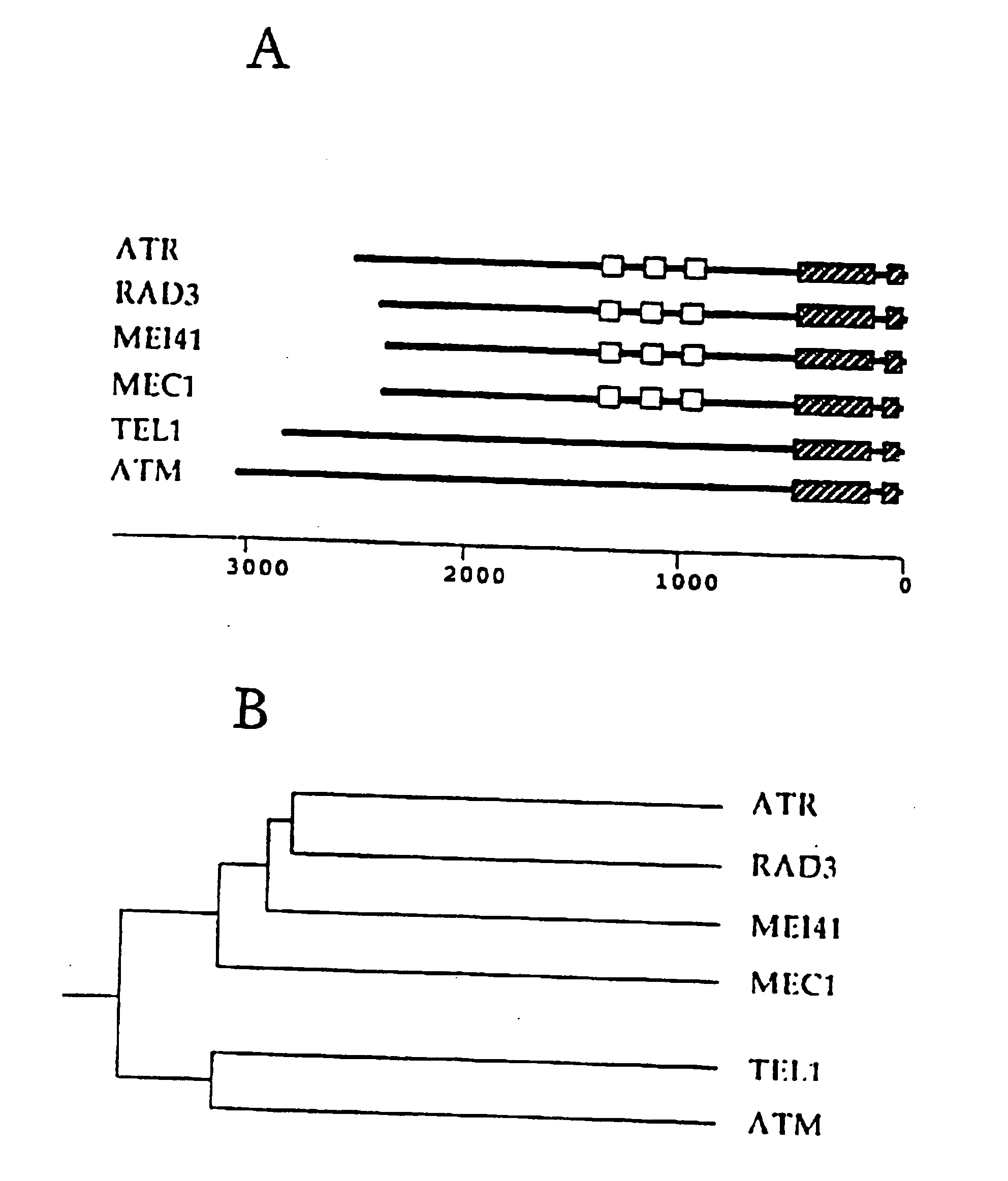
This invention relates to a class of checkpoint genes and their polypeptide products which control progression through the cell cycle in eukaryotic cells. In particular, this invention relates to Schizosaccharomyces pombe rad3 gene, to its human homologue (ATR), and to their encoded proteins. The invention further relates to assay methods for selecting compounds which modulate the activity of the polypeptide products of these checkpoint genes and the use of the selected compounds in anticancer therapy.
Owner:ICOS CORP
Method for therapeutic angiogenesis
InactiveUS20120107297A1Level and activity can be reducedImprove stabilityPeptide/protein ingredientsLigasesAnticarcinogenAngiogenesis Inducing Agents
The present invention relates to the E2EPF UCP-VHL interaction and the uses thereof, more precisely a method for increasing or reducing VHL activity or level by regulating UCP activity or level to inhibit cancer cell proliferation or metastasis or to increase angiogenesis. The inhibition of UCP activity is accomplished by any UCP activity inhibitor selected from a group consisting of a small interfering RNA (RNAi), an antisense oligonucleotide, and a polynucleotide complementarily binding to mRNA of UCP, a peptide, a peptide mimetics and an antibody, and a low molecular compound. In the meantime, the increase of angiogenesis is accomplished by the following mechanism; UCP over-expression is induced by a gene carrier and thus endogenous VHL is reduced, leading to the stabilization of HIF-1α which enhances VEGF activation based on the HIF-1α stabilization. The method for regulating UCP activity or level results in the increase or decrease of VHL activity or level, so that it can be applied to the development of an anticancer agent and an angiogenesis inducer.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Establishment method of positive screening system based on gene knockout cells
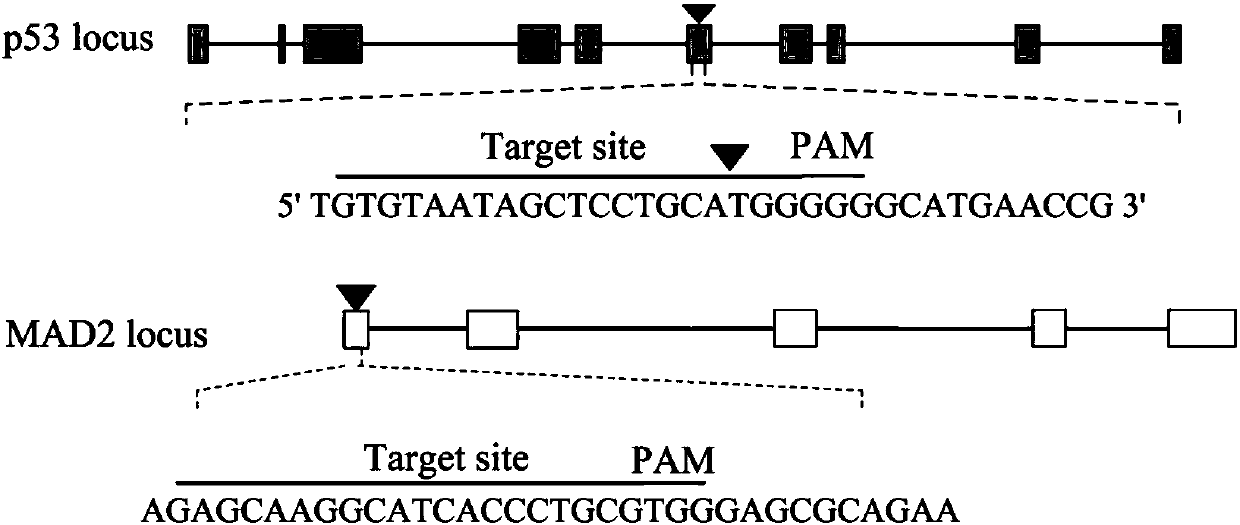
The invention belongs to the technical field of zoobiology, and relates to an establishment method of a positive screening system based on gene knockout cells; on the basis of the established system,p53 and MAD2 genes are knocked out in NIH / 3T3 cells, and the cells, which undergo gene knockout, are subjected to positive screening. According to the establishment method, target sequences of the sixth exon of the p53 gene and the first exon of the MAD2 gene are subjected to site-directed knockout in an NIH / 3T3 gene line, and in the combination with a green fluorescent protein gene and a puromycin gene, the cells, which undergo p53 and MAD2 gene knockout, are subjected to positive screening; and by virtue of the established positive screening system, the p53 and MAD2 genes are simultaneouslyedited in living mouse liver, so that a mouse liver cancer model is finally obtained. The method provided by the invention lays a foundation for related researches on editing the p53 and MAD2 genes ina living mouse.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Anticancer vaccine and diagnostic methods and reagents
Owner:UNIVERSITY OF PITTSBURGH +1
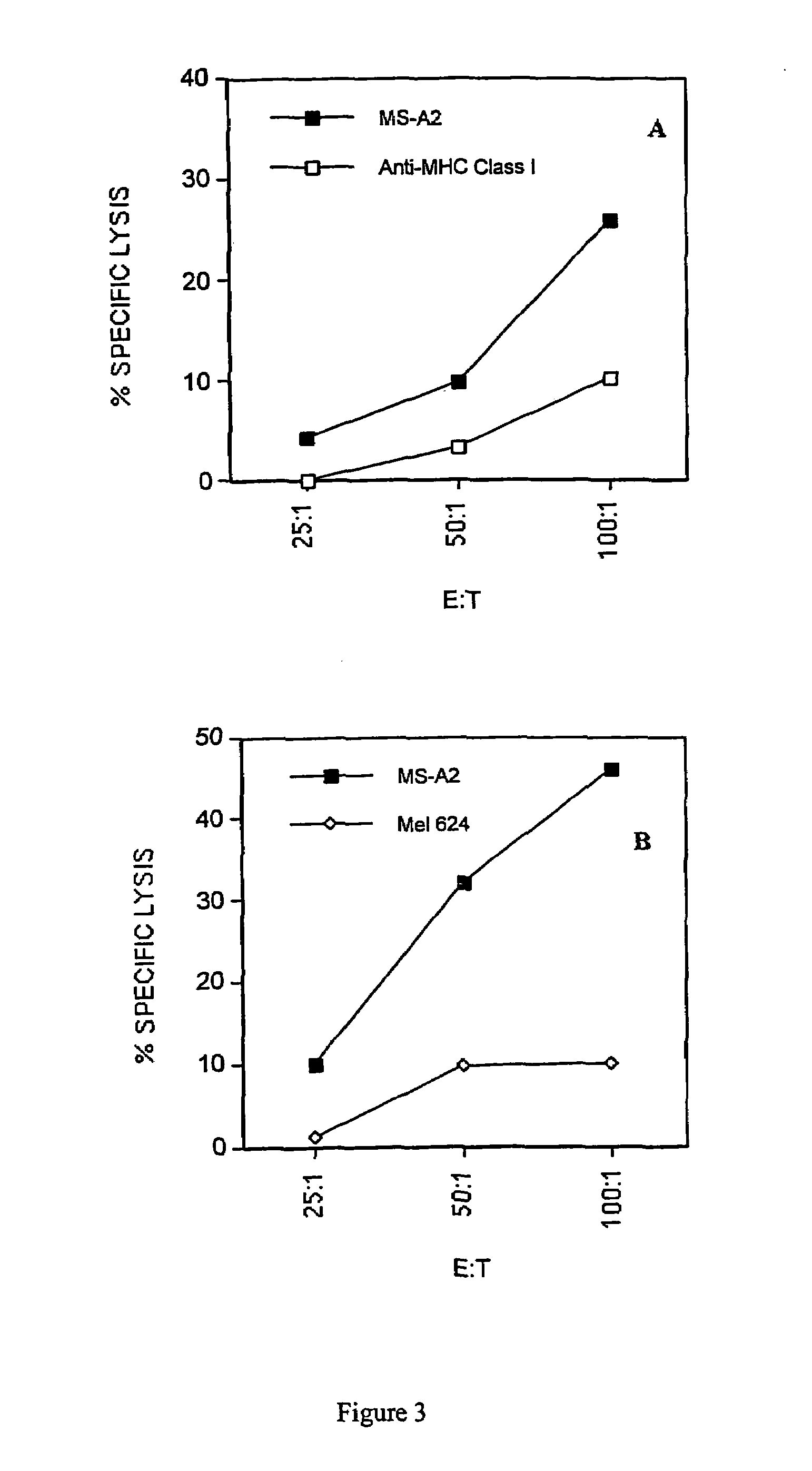
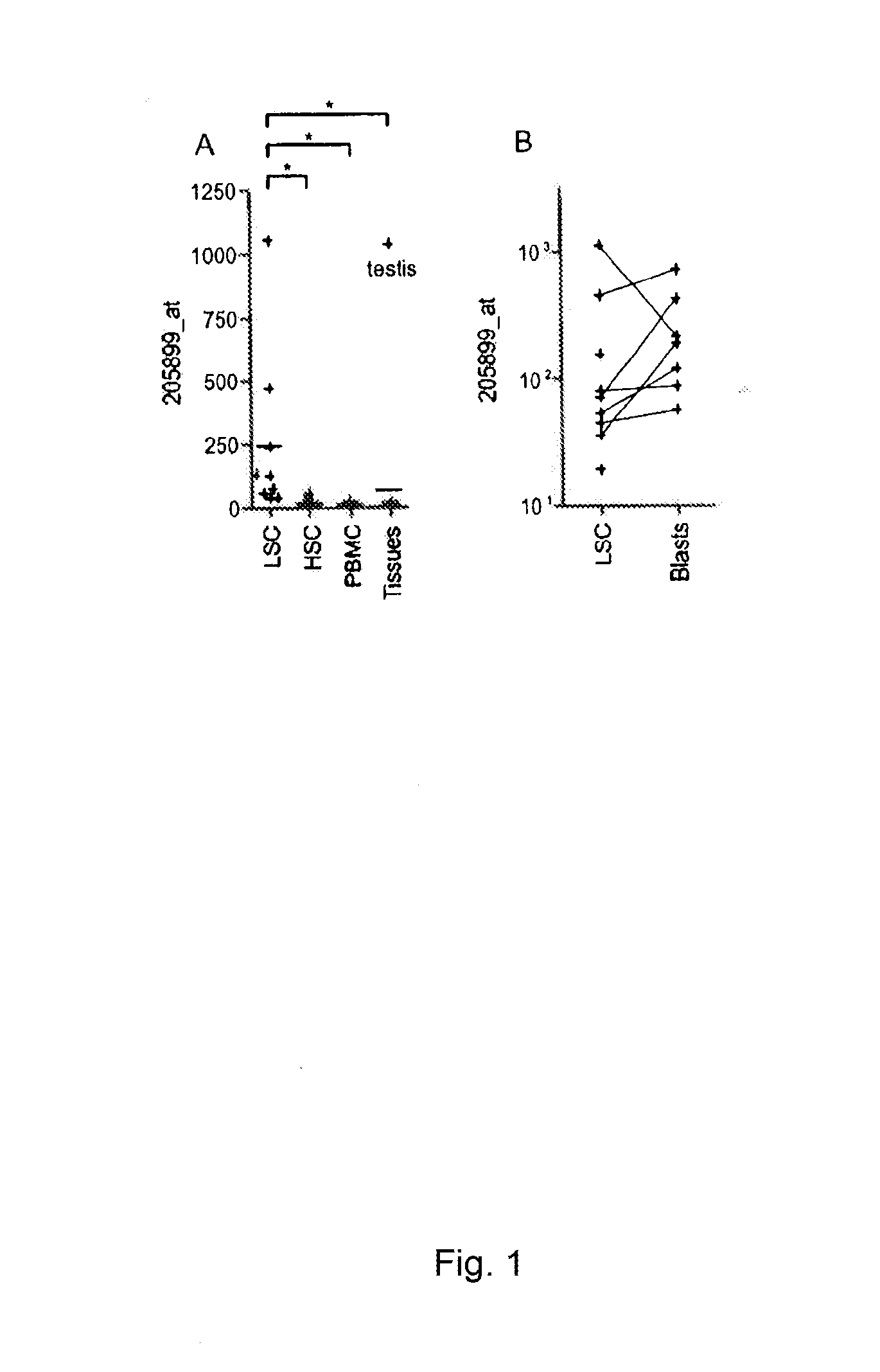
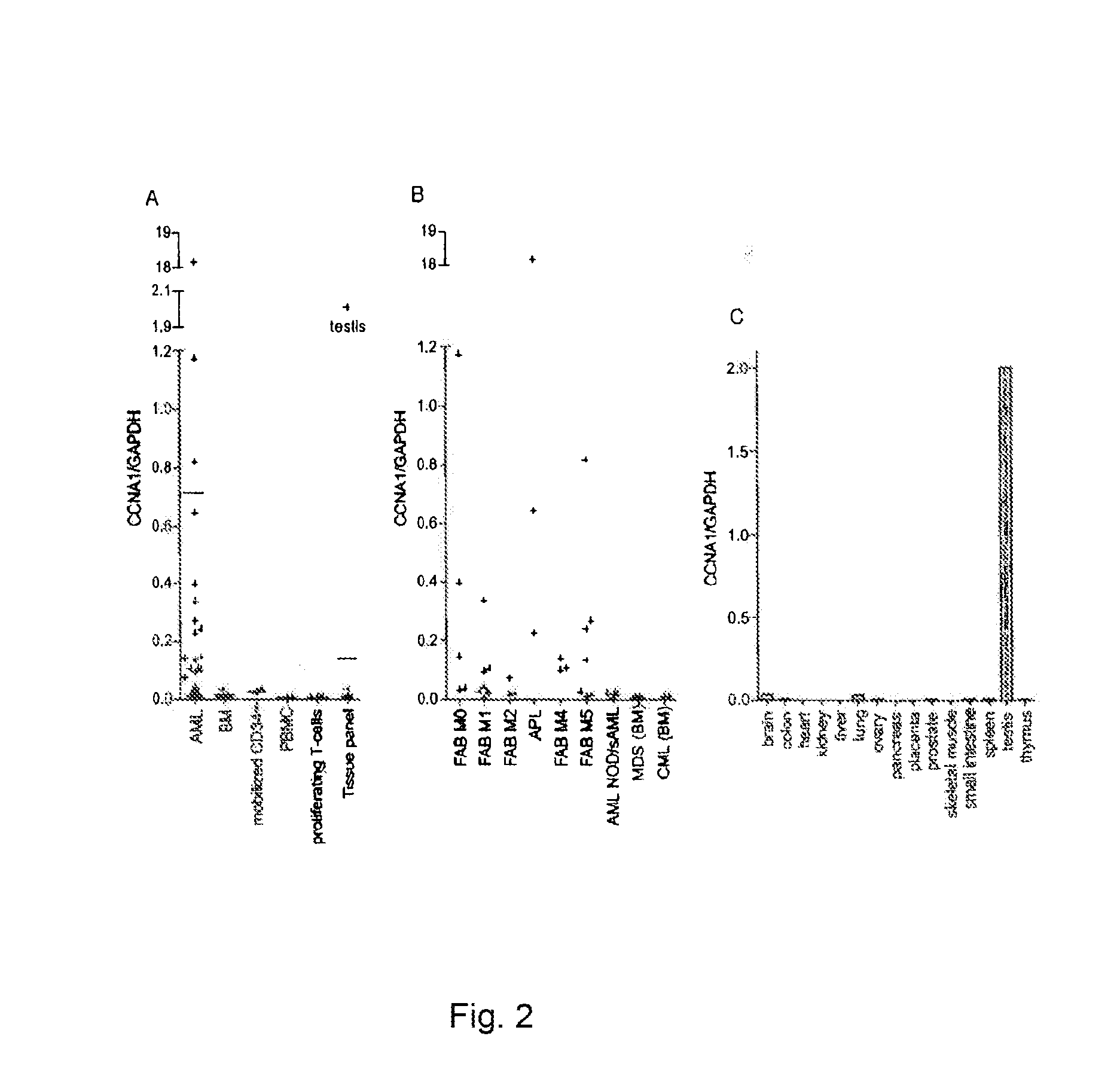
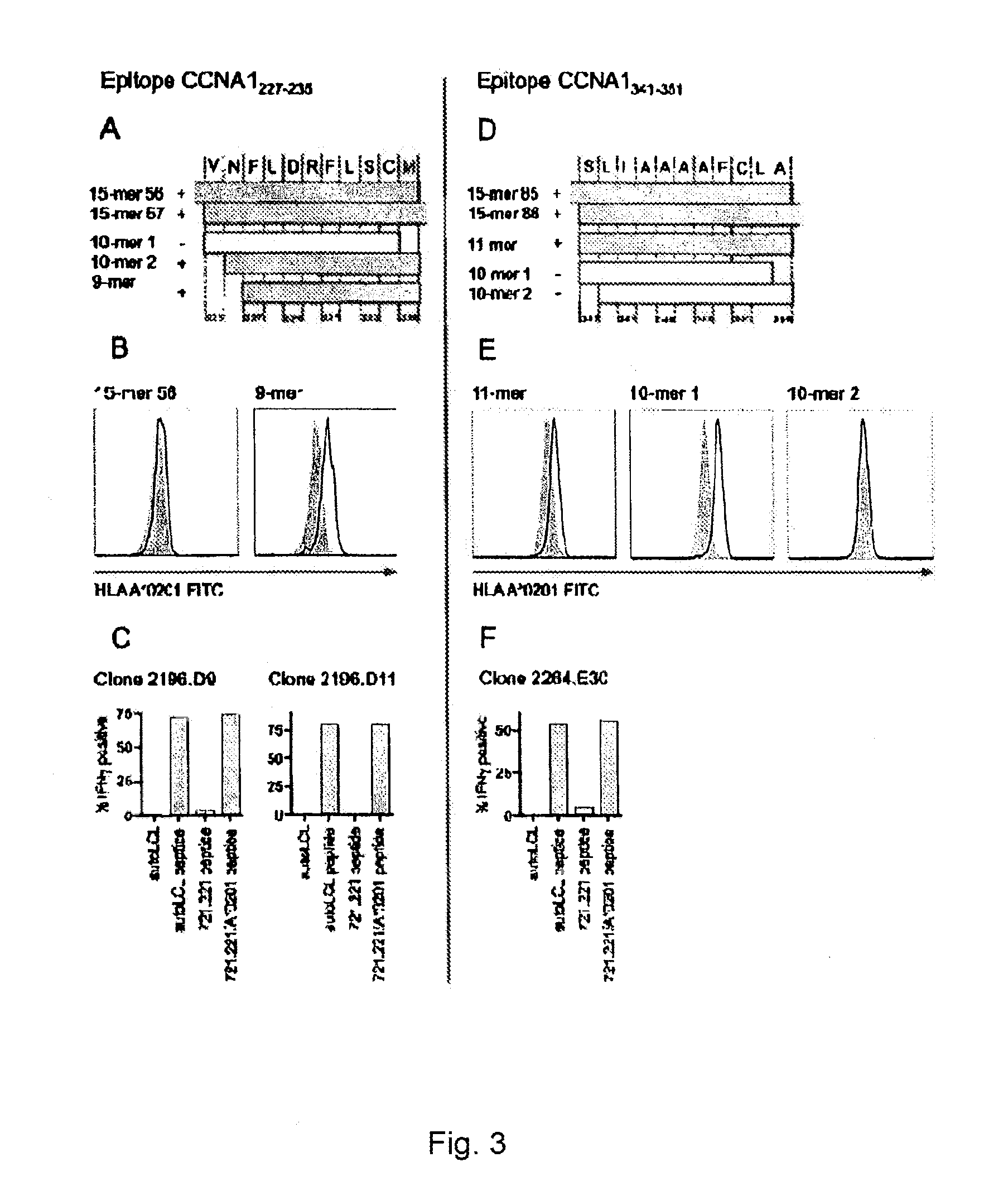
Cyclin a1-targeted t-cell immunotherapy for cancer
Compositions and methods are provided for eliciting antigen-specific T-cell responses against human cyclin A1 (CCNA1), which is herein identified as a leukemia-associated antigen based on its overexpression in acute myeloid leukemia (AML) including leukemia stem cells (LSC) and in immunologically privileged testis cells, but not in other normal cell types. CCNA1-derived peptide epitopes that are immunogenic for T-cells including CTL are disclosed, as are immunotherapeutic approaches using such peptides for vaccines and generation of adoptive transfer therapeutic cells.
Owner:FRED HUTCHINSON CANCER CENT
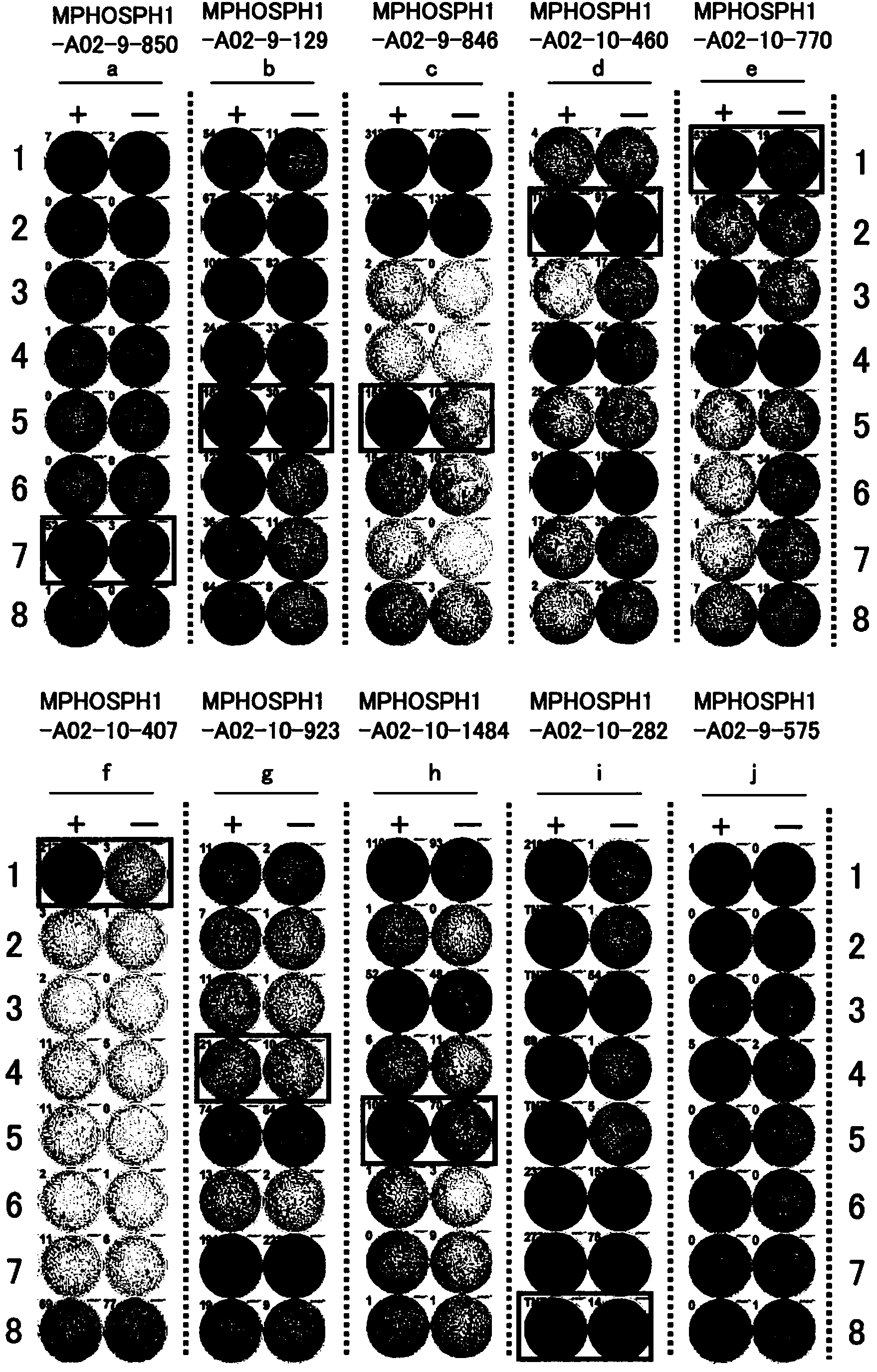
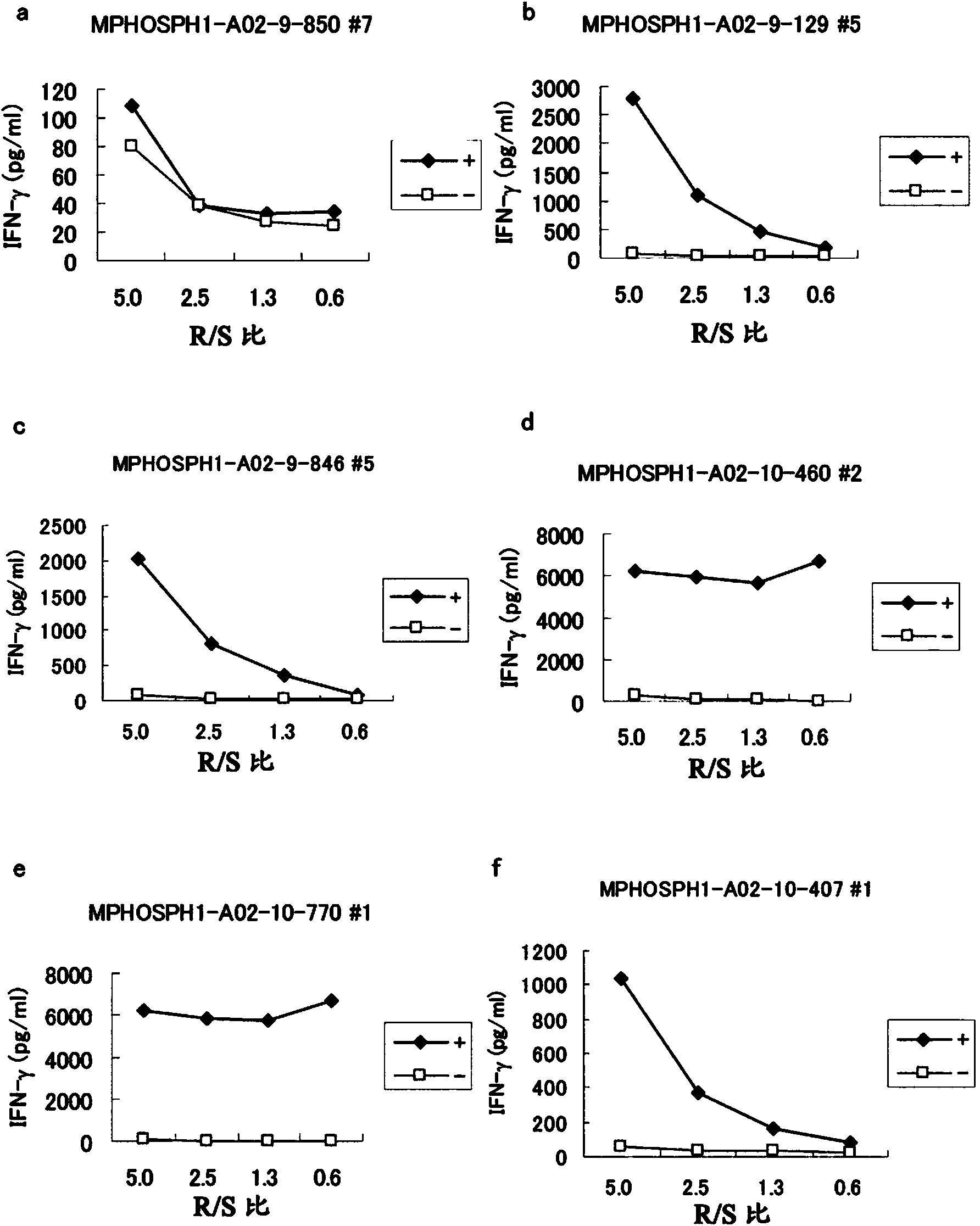
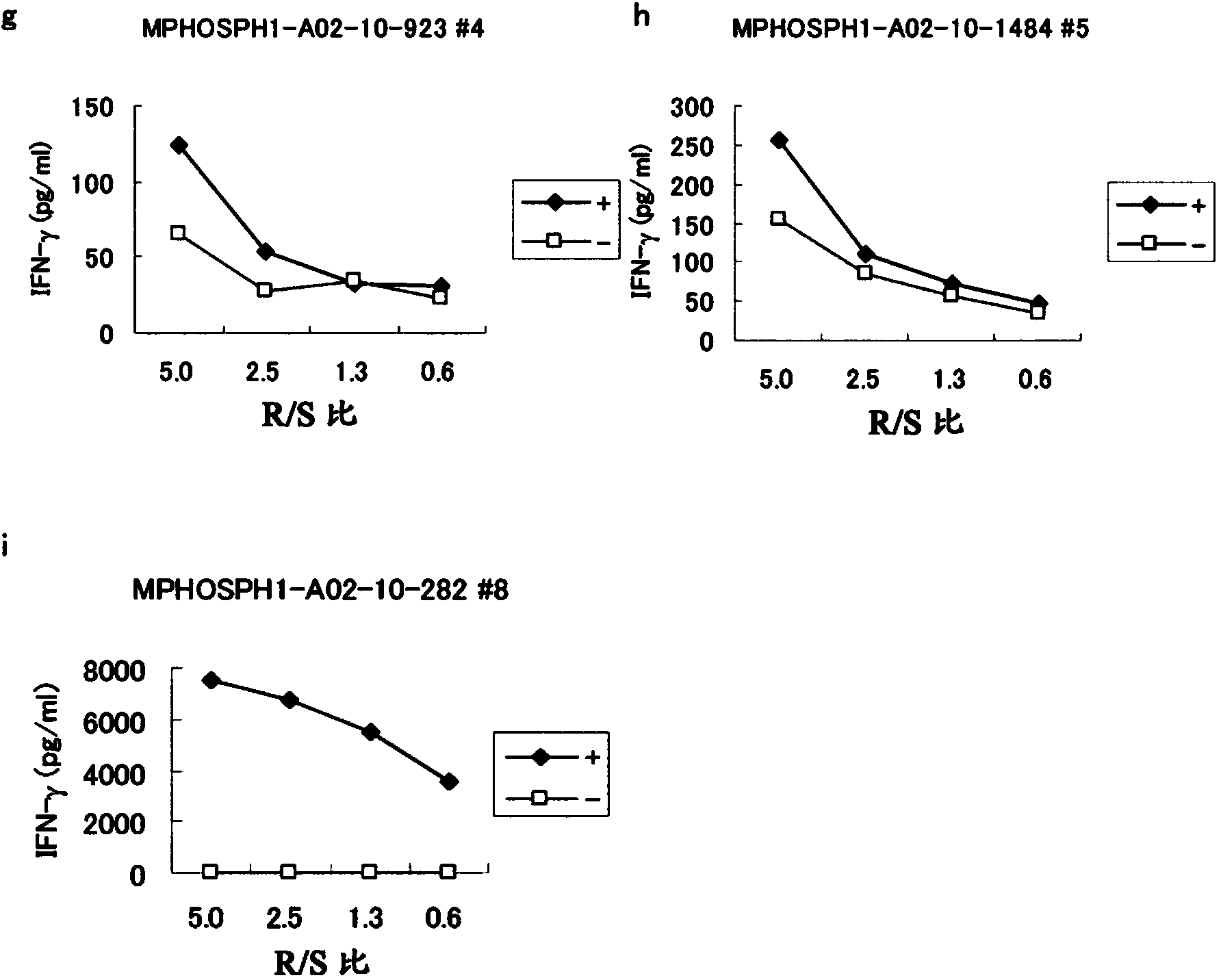
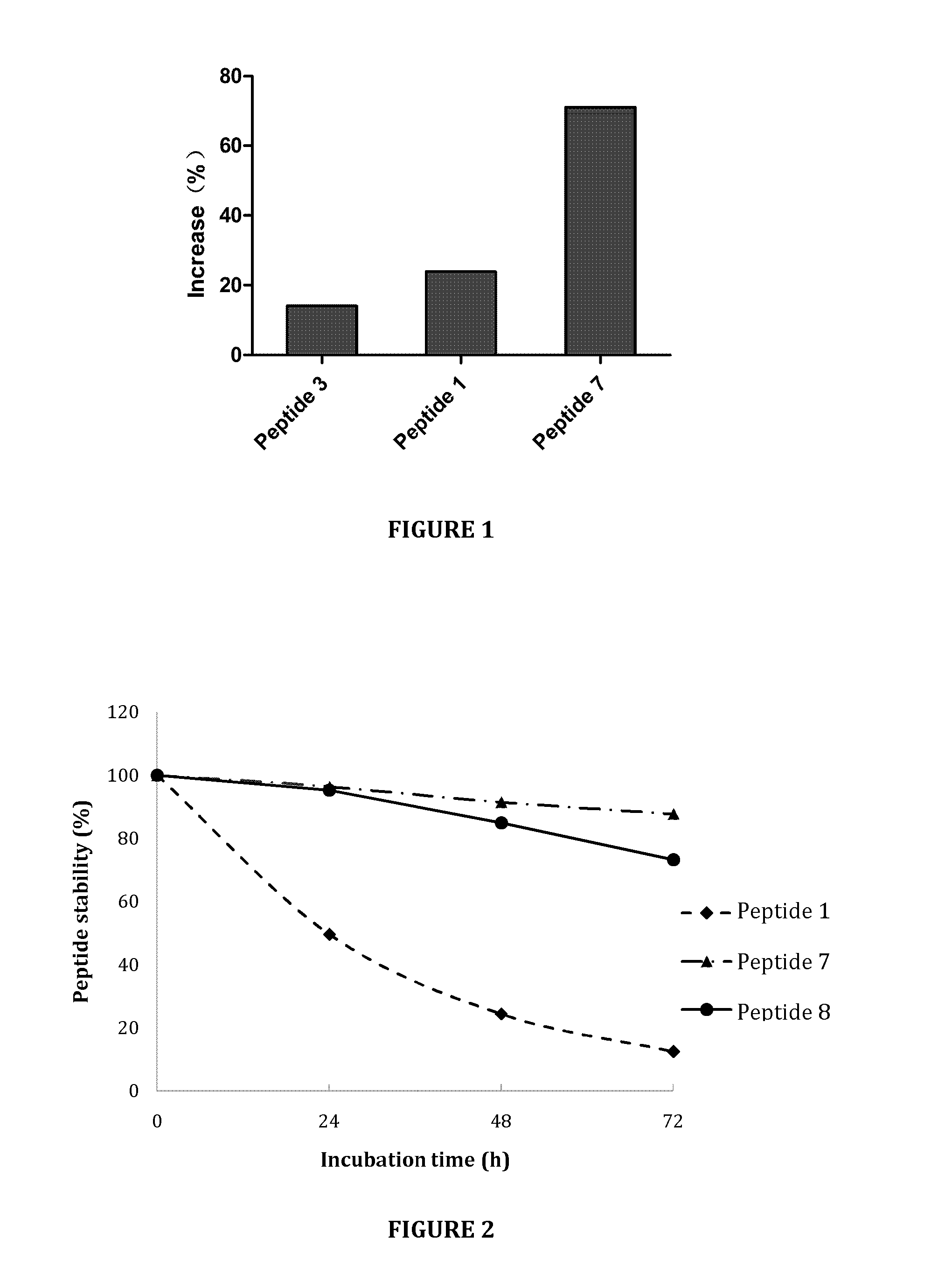
Mphosph1 peptides and vaccines including the same
As discussed in greater detail herein, isolated epitope peptides derived from MPHOSPH1 bind to an HLA antigen and induce cytotoxic T lymphocytes (CTL) and thus are suitable for use in the context of cancer immunotherapy, more particularly cancer vaccines. The inventive peptides encompass both the above-mentioned MPHOSPH1-derived amino acid sequences and modified versions thereof, in which one, two, or several amino acids are substituted, deleted, inserted or added, provided such modified versions retain the requisite CTL inducibility of the original sequences. Further provided are polynucleotides encoding any of the aforementioned peptides as well as pharmaceutical agents or compositions that include any of the aforementioned peptides or polynucleotides. The peptides, polynucleotides, and pharmaceutical agents or compositions of this invention find particular utility in either or both of the treatment and prevention of cancers and tumors, including, for example, bladder cancer, breast cancer, cervical cancer, cholangiocellular carcinoma, CML, colorectal cancer, gastric cancer, NSCLC, lymphoma, osteosarcoma, prostate cancer, renal cancer and soft tissue tumor.
Owner:ONCOTHERAPY SCI INC
Compositions and methods of using islet neogenesis peptides and analogs thereof
ActiveUS20150203538A1Promoting neuroprotectionPromote nerve regenerationNervous disorderPeptide/protein ingredientsDisease causeInflammation
The invention provides peptides and analogs of INGAP and HIP peptides. The peptides and analogs can be used in methods for treating various diseases and conditions. Such diseases and conditions can include impaired pancreatic function, treating a metabolic disease, for example, diabetes, both type 1 and type 2 diabetes, islets induction, expansion and proliferation for trans plantation, promoting neuroprotection or nerve regeneration, promoting liver regeneration or inhibiting inflammation.
Owner:SHENZHEN HIGHTIDE BIOPHARM
Inhibitor of cell proliferation and methods of use thereof
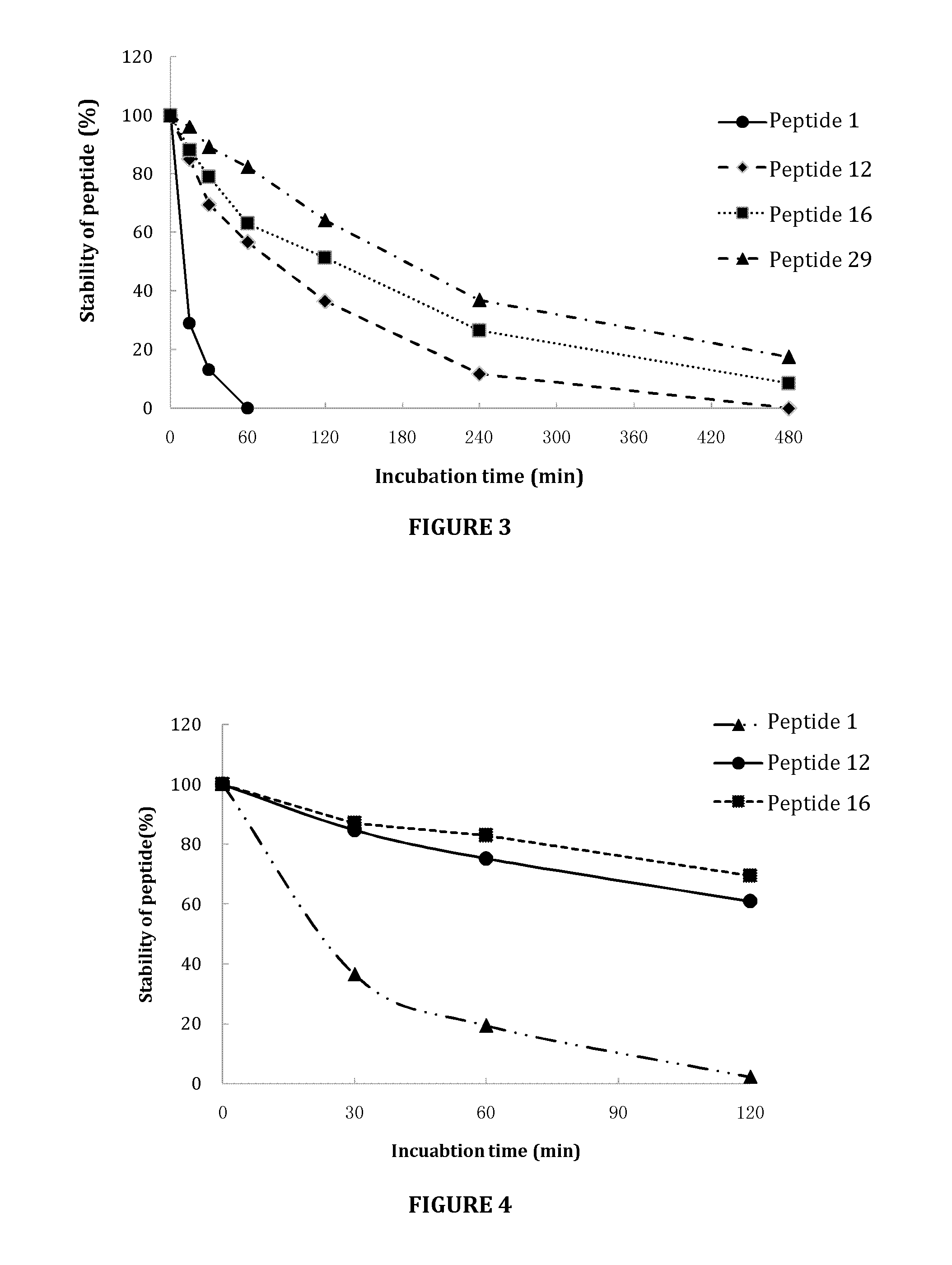
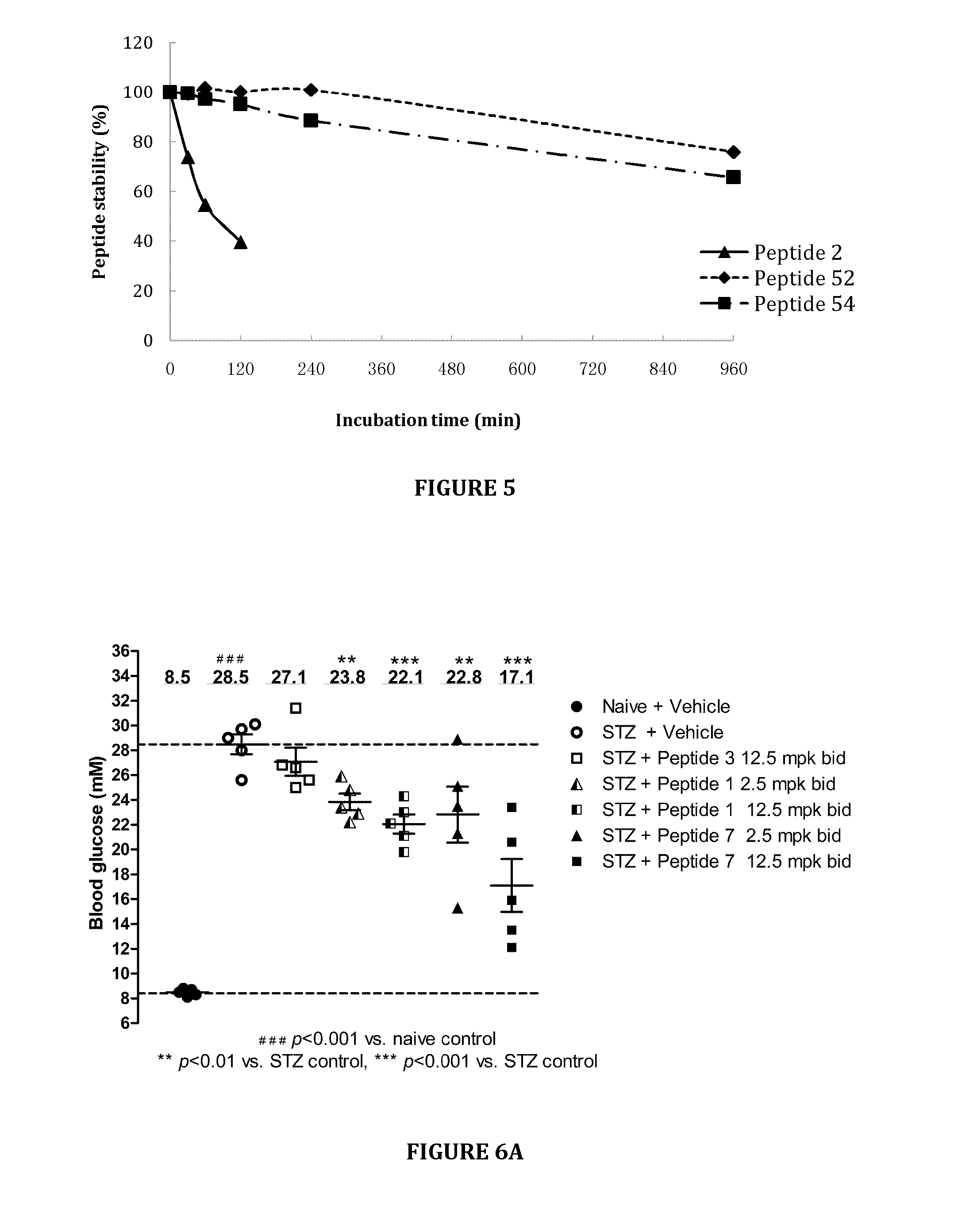
An chimerical polypeptide that arrests proliferating cells in mitosis is provided. In general the polypeptide has an N-terminal transit peptide, such as HIV-1 Tat, and a C-terminal cell-cycle effector, such as a G2 / M cyclin or a cytostatic factor. A polynucleotide under the control of a heterologous promoter that encodes a polypeptide that arrests cells in mitosis is also provided. The polynucleotide may, for example, encode a G2 / M cyclin. Pharmaceutical compositions comprising the agent that inhibits transit through mitosis is provided. A method of treating patients suffering from a hyperplasia, such as cancer, psoriasis or benign prostate hyperplasia is provided.
Owner:NUCLEUS REMODELING
Markers for prostate cancer
InactiveUS6972170B1Extend your lifeProlong the life-span of the patientPeptide/protein ingredientsGenetic material ingredientsProstate cancerProstate hyperplasia
This invention provides a method for determining the aggressiveness of a prostate carcinoma comprising: (a) obtaining a sample of the prostate carcinoma; and (b) detecting the presence of p27 protein in the prostate carcinoma, the absence of p27 indicating that the prostate carcinoma is aggressive. This invention also provides a method for diagnosing a beign prostate hyperplasia comprising: (a) obtaining an appropriate sample of the hyperplasia; and (b) detecting the presence of the p27 RNA, a decrease of the p27 RNA indicating that the hyperplasia is beign. This invention provides various uses of p27 in prostate cancer. Finally, this invention also provides different marker for prostate cancer.
Owner:SLOAN KETTERING INST FOR CANCER RES
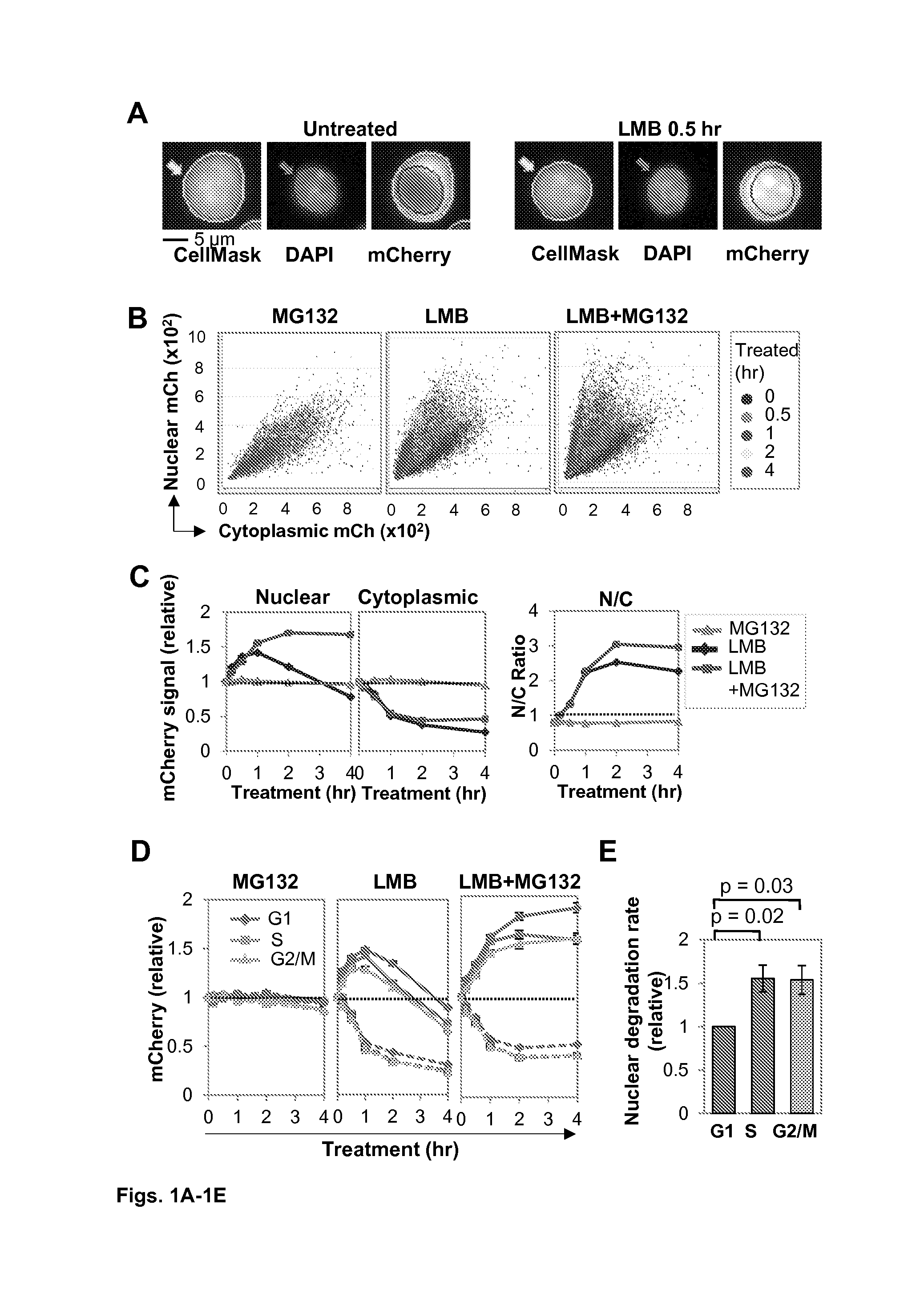
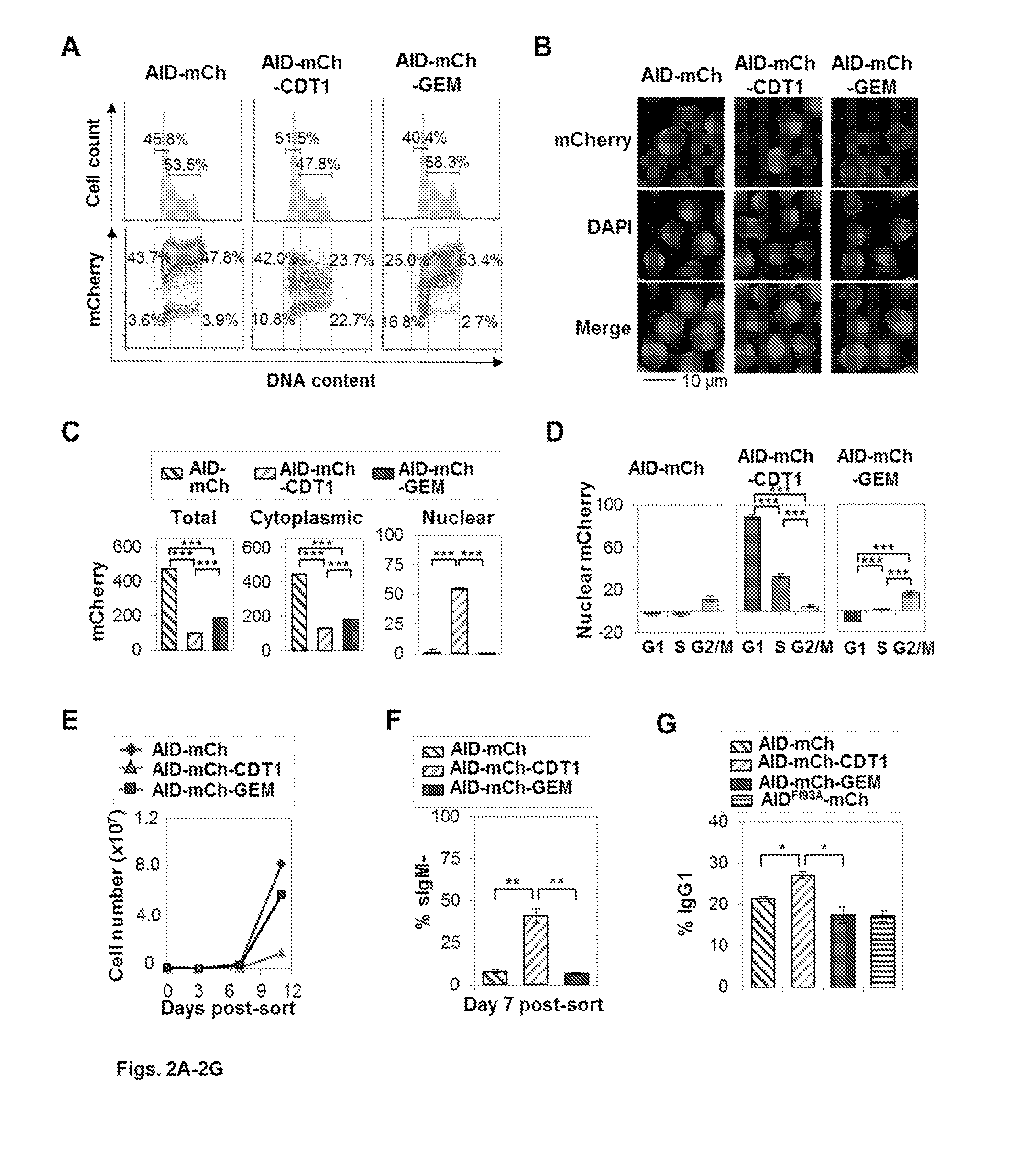
Restricting nuclear protein to specific phases of the cell cycle
InactiveUS20160369258A1Accelerates mutagenesisPrevent nuclear exportHydrolasesAntibody mimetics/scaffoldsT cellImmunotherapy
The present invention relates generally to mutagenesis of target genes that enhances the natural mutagenic capabilities of adaptive immune cells by providing a chimeric construct that exploits the ability of molecules such as AID to stimulate diversification and the ability of a second molecule to restrict nuclear activity of the molecules and / or protect cell viability. The invention provides a method for stimulating diversification in expressed genes, such as antibody genes, using polypeptides whose nuclear activity is restricted to specific phases of the cell cycle. This method can be coupled with selection to identify B cell clones that produce, for example, antibodies of high affinity or specificity, or for developing T cells for immunotherapy. The invention provides an improved means of developing a repertoire of variant immunoglobulins and other polypeptides.
Owner:UNIV OF WASHINGTON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com