Establishment method of positive screening system based on gene knockout cells
A technology for knocking out genes and establishing methods, applied in chemical instruments and methods, cell cycle regulatory proteins, biochemical equipment and methods, etc., can solve problems such as cell aneuploidy, tumors, and chromosomal instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
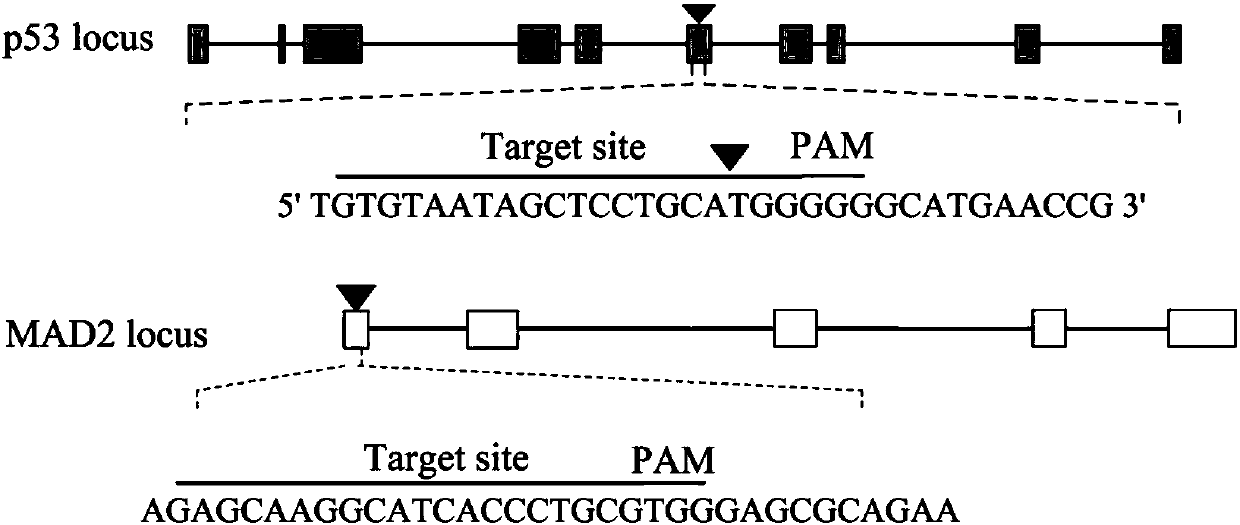
[0029] Example 1 Construction of a positive screening system for gene knockout cells
[0030] According to the following experimental steps:
[0031] 1) NCBI (https: / / www.ncbi.nlm.nih.gov / ) to query mouse p53 and MAD2 gene sequences, use CHOPCHOP website (https: / / chopchop.rc.fas.harvard.edu / ) to design and use To knock out the target recognition sequences of MAD2 and p53 genes, select the 20bp target sequence of the sixth exon of p53 gene and the first exon of MAD2 gene, and synthesize oligos in Invitrogen (Shanghai) according to the designed 20bp recognition sequence. Nucleotide
[0032] 2) The 20bp target sequence oligonucleotides of the sixth exon of p53 gene and the first exon of MAD2 gene were ligated with pCas9-GFP-puro plasmid after annealing, denaturation and hybridization, and then transformed into the large intestine by heat shock at 42℃ Bacillus competent, shake at 220rpm / min at 37℃ for 1h, take 100μl of bacterial solution and spread it on the ampicillin (Amp) LB medium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com