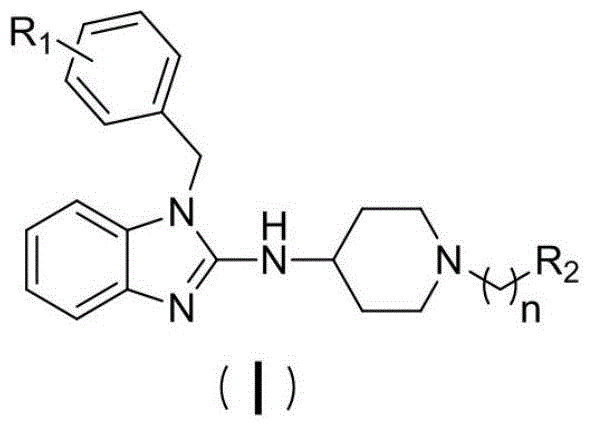
A kind of small molecule fluorescent probe of benzimidazole herg potassium ion channel and its preparation method and application
A technology of potassium ion channels and benzimidazoles, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of few applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Preparation of coumarin-based fluorophore probes:
[0033]
[0034] Concrete synthetic route is as follows:
[0035]
[0036] (1) Preparation of Intermediate 1:
[0037] Add 30ml of acetonitrile and potassium hydroxide (2.62g, 46.6mmol) to 2-chlorobenzimidazole (5g, 32.7mmol), heat to 80°C, stir for 30min, the solution is clear; after cooling to room temperature, add p-fluorobenzyl bromide ( 9.29g, 49.1mmol), heated to 80°C and refluxed for 5h, the reaction solution was a white turbid solution; the reaction solution was extracted three times with dichloromethane (100mL×3), washed with distilled water, combined organic layers, dried over anhydrous magnesium sulfate, pumped Filter and concentrate to obtain a white solid, which is recrystallized with acetone / petroleum ether, refrigerated, suction filtered, and dried to obtain a white solid with a yield of 91.1%. 1H-NMR (300MHz, DMSO): δ=7.64(d, J=6.6Hz, 2H), 7.32-7.24(m, 4H), 7.19(t, J=4.9Hz, 2H), 5.5...
Embodiment 2
[0053] Example 2: Preparation of naphthalene diimide fluorophore probes
[0054]
[0055] Concrete synthetic route is as follows:
[0056]
[0057] In the synthetic route of this example, the preparation method of intermediate 1-3 is the same as that of intermediate 1-3 in Example 1.
[0058] (1) Preparation of Intermediate 4:
[0059] Add 4 mL of glacial acetic acid and 20 mL of pyridine to 4-amino-1,8-naphthalene diimide (2 g, 9.3 mmol), and reflux for 1 h. Then 30mL of acetic anhydride was added, and the reaction was refluxed for 5h. After the reaction solution was cooled to room temperature, it was poured into 200 mL of ice water, filtered with suction, and the filter cake was washed with water to obtain a crude brown solid with a yield of 71.3%.
[0060] (2) Preparation of intermediate 5:
[0061] Intermediate 4 (1.7g, 6.66mmol) and 4-amino-1-butanol (0.71g, 7.96mmol) were dissolved in absolute ethanol and refluxed for 5h. The reaction solution was concentrated...
Embodiment 3
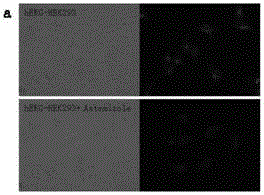
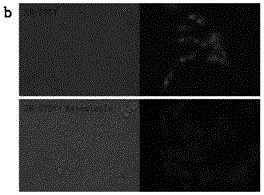
[0066] Example 3: Determination of Optical Activity
[0067] Table 1: Optical characteristics of probe molecules
[0068]
[0069]
[0070] Note: All the above optical properties were measured in phosphate buffer at pH = 7.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com