Method for preparing proton-exchange membrane fuel cell oxygen reduction catalyst based on PtNi (111) octahedral single crystal nanoparticles
A proton exchange membrane, fuel cell technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc. Shortening lattice spacing, avoiding agglomeration, and good monodispersity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
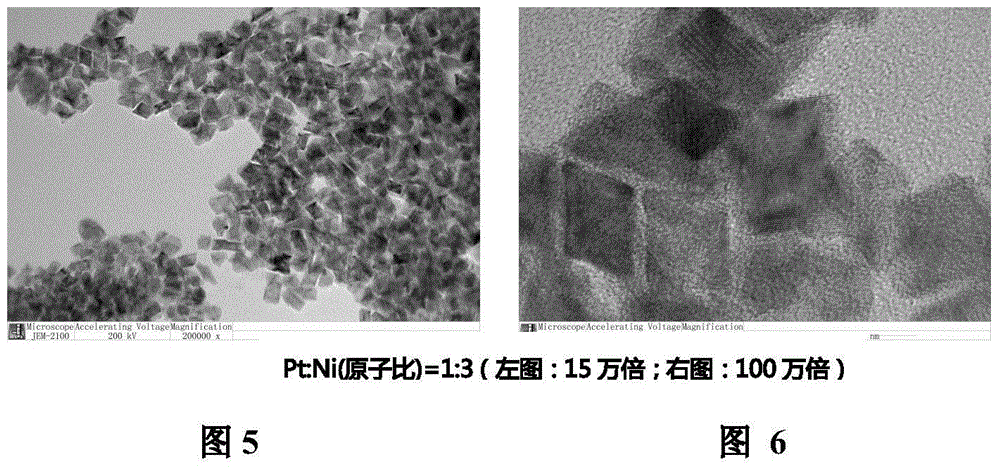
[0025]Example 1: Weigh 39.3 mg of platinum acetylacetonate and 77.1 mg of nickel acetylacetonate, and put them into 50 mL of N,N-dimethylformamide to fully dissolve, wherein the atomic ratio of Pt:Ni is 1:3. The mixture was placed in an autoclave, heated in an oven at 120°C for 42 hours, the resulting solution was cooled to room temperature and then 50 mL of n-hexane was added, ultrasonicated for 3 hours and the temperature of the water bath was controlled not to exceed 30°C. Then the above-mentioned highly dispersed sol was added dropwise to the 28.2 mg conductive carbon black solution in the form of back titration, after stirring for 48 hours, static filtration, and alternate cleaning with deionized water and ethanol, and finally placed in a vacuum oven at 60 °C for 6 hours. The catalytic oxidation source (ORR) activity value of this product is about 1.50A / mg.
Embodiment 2
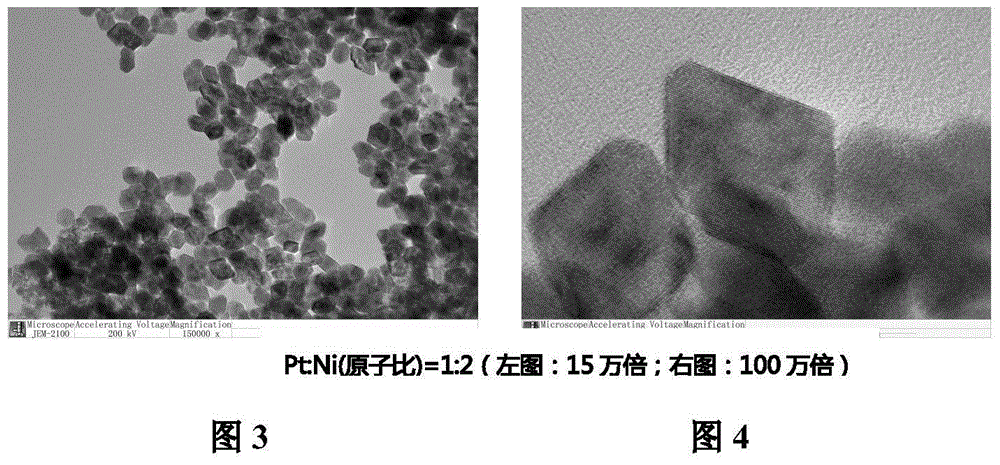
[0026] Example 2: Weigh 39.3 mg of platinum acetylacetonate and 51.4 mg of nickel acetylacetonate, and put them into 37.5 mL of N,N-dimethylformamide to fully dissolve, wherein the atomic ratio of Pt:Ni is 1:2. The mixture was placed in an autoclave, heated in an oven at 120°C for 42 hours, the resulting solution was cooled to room temperature and then 25 mL of n-hexane was added, ultrasonicated for 3 hours and the temperature of the water bath was controlled not to exceed 30°C. Then the above-mentioned highly dispersed sol was added dropwise to 34.0 mg conductive carbon black solution in the form of back titration, stirred for 48 hours, statically filtered, and the resulting product was washed alternately with deionized water and ethanol, and finally placed in a vacuum oven at 60 °C for 6 hours. The resulting product had an oxidative origin (ORR) activity of about 0.95 A / mg.
Embodiment 3
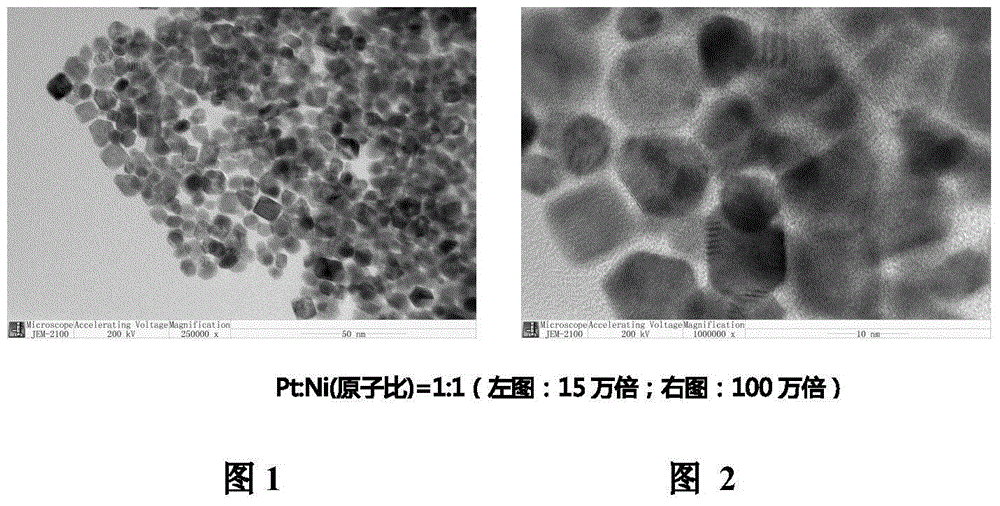
[0027] Example 3: 39.3 mg of platinum acetylacetonate and 25.7 mg of nickel acetylacetonate were weighed and put into 25 mL of N,N-dimethylformamide to fully dissolve, wherein the atomic ratio of Pt:Ni was 1:1. The mixture was placed in an autoclave, heated in an oven at 120°C for 42 hours, the resulting solution was cooled to room temperature and then 50 mL of n-hexane was added, ultrasonicated for 3 hours and the temperature of the water bath was controlled not to exceed 30°C. Then the above-mentioned highly dispersed sol was added dropwise to 40.0 mg conductive carbon black solution in the form of back titration, stirred for 48 hours, filtered statically, and the resulting product was washed alternately with deionized water and ethanol, and finally placed in a vacuum oven at 60 °C for 6 hours. The resulting product had an oxidative origin (ORR) activity of approximately 0.7 A / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com