Silver-containing perovskite type oxygen reduction catalyst, and preparation method thereof
A perovskite type and catalyst technology, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve the problem of low conductivity of catalysts and achieve preparation methods Simple, large specific surface area, high conductivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
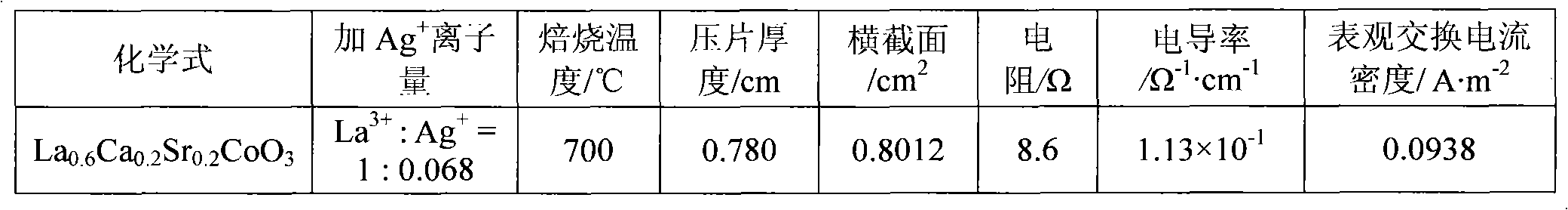
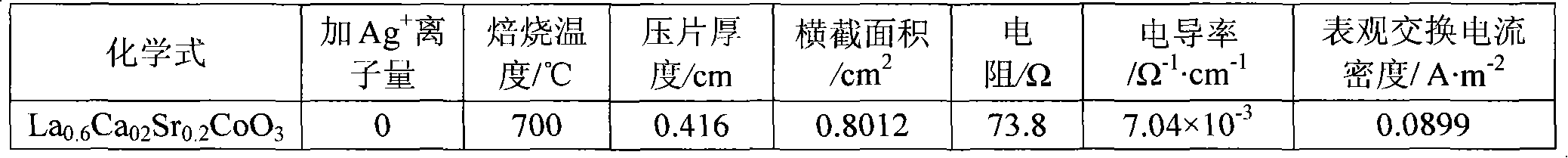
[0013] In the first step, weigh an appropriate amount of calcium nitrate Ca(NO 3 ) 2 4H 2 O (analytical grade), strontium nitrate Sr (NO 3 ) 2 ·6H 2 O, cobalt nitrate Co(NO 3 ) 2 ·6H 2 O (analytical grade), lanthanum nitrate La (NO 3 ) 3 ·6H 2 O (analytical grade) is dissolved in distilled water, then, in this solution, by molar ratio La 3+ : Ag + =1:0.068 Add silver nitrate AgNO 3 (analytical pure), adjust the pH value at 2.0 with nitric acid and ammonia water, then hydrolyze the solution at a constant temperature of 60°C to obtain a sol;
[0014] In the second step, the moisture of the sol was evaporated to dryness to obtain a pink gel, which was aged at room temperature for 48 hours;
[0015] In the third step, the above-mentioned gel aged at room temperature is placed in a muffle furnace, and the temperature is raised from room temperature to 500° C., and the temperature is kept for 8 hours to decompose the residual nitrate in the gel, and then cool naturally ...
Embodiment 2
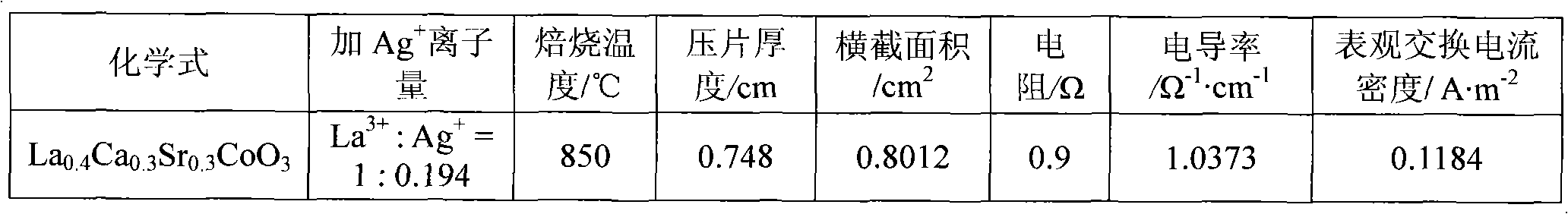
[0026] In the first step, weigh an appropriate amount of calcium nitrate Ca(NO 3 ) 2 4H 2 O (analytical grade), strontium nitrate Sr (NO 3 ) 2 ·6H 2 O, cobalt nitrate Co(NO 3 ) 2 ·6H 2 O (analytical grade), lanthanum nitrate La (NO 3 ) 3 ·6H 2 O (analytical grade) is dissolved in distilled water, then, in this solution, by molar ratio La 3+ : Ag + =1:0.194 Add silver nitrate AgNO 3 (analytical pure), adjust the pH value at 1 with nitric acid and ammonia water, and then the solution is hydrolyzed at a constant temperature of 90°C to obtain a sol;
[0027] In the second step, the moisture of the sol was evaporated to dryness to obtain a pink gel, which was aged at room temperature for 12 hours;
[0028] In the third step, the above-mentioned gel aged at room temperature was placed in a muffle furnace, and the temperature was raised from room temperature to 600° C., and kept for 6 hours to decompose the residual nitrate in the gel, and then cooled naturally to obtain...
Embodiment 3
[0035] In the first step, weigh an appropriate amount of calcium nitrate Ca(NO 3 ) 2 4H 2 O (analytical grade), strontium nitrate Sr (NO 3 ) 2 ·6H 2 O, cobalt nitrate Co(NO 3 ) 2 ·6H 2 O (analytical grade), lanthanum nitrate La (NO 3 ) 3 ·6H 2 O (analytical grade) is dissolved in distilled water, then, in this solution, by molar ratio La 3+ : Ag + =1:0.027 Add silver nitrate AgNO 3 (analytical pure), adjust the pH value at 3.0 with nitric acid and ammonia water, then the solution is hydrolyzed at a constant temperature of 40°C to obtain a sol;
[0036] In the second step, the moisture of the sol was evaporated to dryness to obtain a pink gel, which was aged for 72 hours at room temperature;
[0037] In the third step, the above-mentioned gel aged at room temperature was placed in a muffle furnace, and the temperature was raised from room temperature to 650° C., and kept for 3 hours to decompose the residual nitrate in the gel, and then cooled naturally to obtain a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com