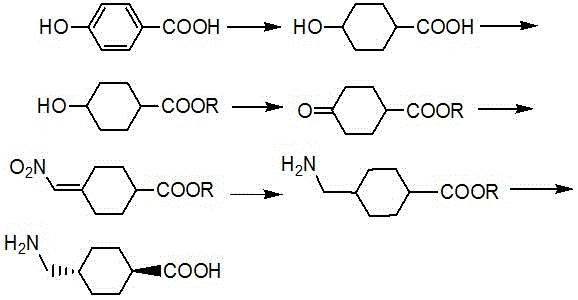
Preparation method of trans-4-aminomethylcyclohexanecarboxylic acid
A technology of aminomethylcyclohexyl formic acid and aminomethylcyclohexyl, which is applied in the field of pharmaceutical synthesis, can solve the problems of high price and high production cost, and achieves the effects of simple operation, low cost, broad development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment one: the preparation of trans-4-aminomethyl cyclohexyl formic acid (synthetic route such as figure 1 shown).
[0036] (1) Catalytic hydrogenation of 4-hydroxybenzoic acid: Add 100g of 4-hydroxybenzoic acid, 500mL of water, 50g of sodium hydroxide and 10g of Raney nickel into the hydrogenation kettle, and pass in hydrogen to remove the air in the kettle. React at 80°C and a pressure of 5MPa, stop the reaction until no more hydrogen is absorbed, cool the material, filter, adjust the filtrate to pH = 2 with dilute sulfuric acid, filter the precipitated solid, and obtain 4-hydroxycyclohexyl formic acid. Purified directly for the next reaction.
[0037] (2) Esterification of 4-hydroxycyclohexylcarboxylic acid: add 4-hydroxycyclohexylcarboxylic acid, 500mL toluene, 10g p-toluenesulfonic acid and 200g ethanol to the reaction kettle, heat and reflux for 8h under stirring, and cool to 50°C , add 300mL of water, stir and separate the layers, wash the toluene layer o...
Embodiment 2
[0042] Example 2: Preparation of trans-4-aminomethylcyclohexylcarboxylic acid.
[0043] (1) Catalytic hydrogenation of 4-hydroxybenzoic acid: Add 100g of 4-hydroxybenzoic acid, 500mL of water, 60g of sodium hydroxide and 15g of Raney nickel into the hydrogenation kettle, and pass in hydrogen to remove the air in the kettle. React at 90°C and a pressure of 6 MPa, stop the reaction until no more hydrogen is absorbed, cool and discharge, filter, adjust the filtrate to pH = 2 with dilute sulfuric acid, filter the precipitated solid, and obtain 4-hydroxycyclohexyl formic acid. Purified directly for the next reaction.
[0044] (2) Esterification of 4-hydroxycyclohexylcarboxylic acid: Add 4-hydroxycyclohexylcarboxylic acid, 500mL toluene, 10g p-toluenesulfonic acid and 200g ethanol to the reaction kettle, heat and reflux for 10h under stirring, and cool to 50°C , adding 500 mL of water, stirring and separating the layers, washing the toluene layer once with water, and concentrating un...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap