Preparation method of crystal lithium hexafluorophosphate
A technology of lithium hexafluorophosphate and crystals, which is applied in the field of preparation of crystal lithium hexafluorophosphate, can solve the problems of difficulty in guaranteeing product quality, poor quality stability of high-purity lithium fluoride, and high production costs, and achieve low production costs, stable product quality, and three-waste discharge little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0044] Example 1
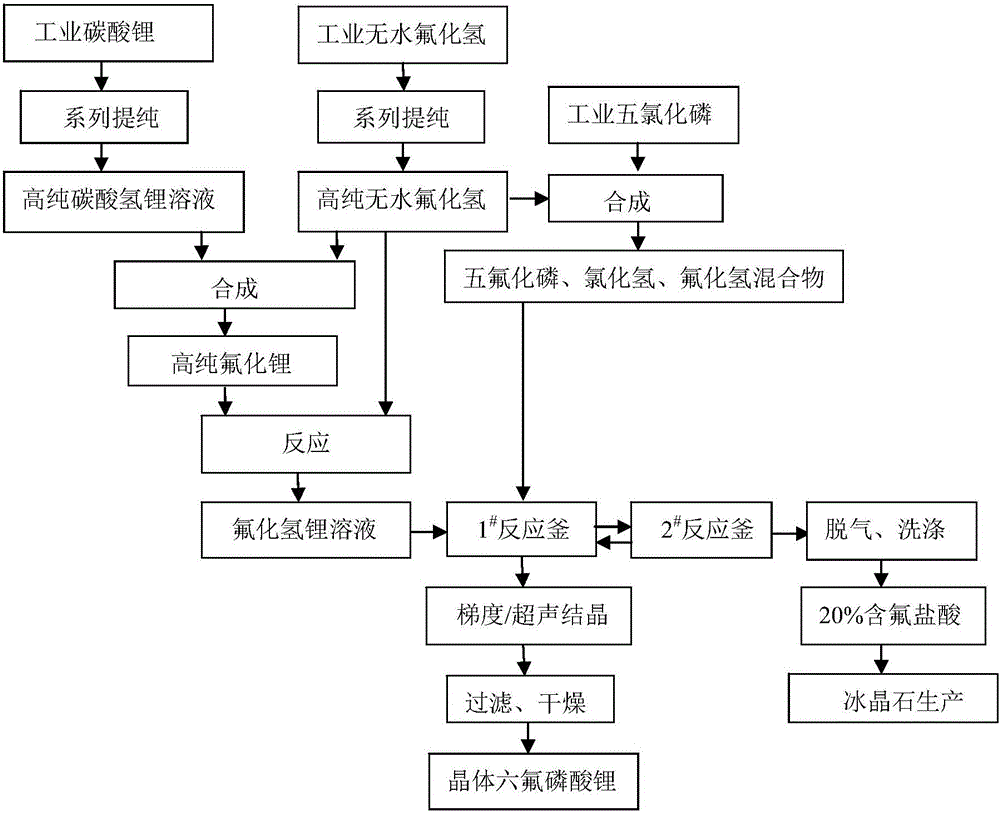
[0045] The preparation method of crystalline lithium hexafluorophosphate in this embodiment, the process flow is as follows figure 1 As shown, including the following steps:
[0046] 1) Pour fluorine gas into 3kg of industrial anhydrous hydrogen fluoride to fully oxidize to obtain a mixed gas; the mixed gas is subjected to two-stage rectification below 0.1Mpa; the temperature of the tower kettle of the first-stage rectification is 20℃, and the outlet temperature is 19℃ ; The temperature of the tower kettle of the secondary rectification is 15℃, and the outlet temperature is 12℃; the hydrogen fluoride after rectification is filtered through a 0.2μm microporous membrane and a secondary 0.05μm microporous membrane to remove the tiny amounts of industrial hydrogen fluoride raw materials Particulate impurities, 2.88kg of high-purity anhydrous hydrogen fluoride (purity ≥99.999wt%);
[0047] Mix 0.4kg of industrial lithium carbonate and water into a 3wt% slurry, and the...
Example Embodiment
[0054] Example 2
[0055] The preparation method of crystalline lithium hexafluorophosphate in this embodiment has the same process flow as that of embodiment 1, including the following steps:
[0056] 1) Pass fluorine gas into 16kg of industrial anhydrous hydrogen fluoride to fully oxidize to obtain a mixed gas; the mixed gas is subjected to two-stage rectification below 0.1Mpa; the temperature of the tower kettle of the first-stage rectification is 35℃ and the outlet temperature is 25℃ ; The temperature of the tower kettle of the secondary rectification is 25℃, and the outlet temperature is 18℃; the hydrogen fluoride after rectification is filtered through the first-level 0.2μm microporous membrane and the second-level 0.05μm microporous membrane to remove the tiny amounts of industrial hydrogen fluoride raw materials Granular impurities, 14.65kg of high-purity anhydrous hydrogen fluoride (purity ≥99.999wt%) is prepared;
[0057] A 3wt% slurry was prepared by 0.8kg industrial lith...
Example Embodiment
[0064] Example 3
[0065] The preparation method of crystalline lithium hexafluorophosphate in this embodiment has the same process flow as that of embodiment 1, including the following steps:
[0066] 1) Pass fluorine gas into 20kg of industrial anhydrous hydrogen fluoride to fully oxidize to obtain a mixed gas; carry out the two-stage rectification of the mixed gas below 0.1Mpa; among them, the temperature of the tower kettle of the first-stage rectification is 20℃~35℃, and the outlet The temperature is 19~25℃; the temperature of the second-stage distillation column is 15℃~25℃, and the outlet temperature is 12~18℃; the hydrogen fluoride after rectification is passed through the first-stage 0.2μm microporous membrane and the second-stage 0.05μm microporous membrane. Porous membrane filtration to remove tiny particulate impurities in industrial hydrogen fluoride raw materials to obtain 18.2kg of high-purity anhydrous hydrogen fluoride (purity ≥99.999wt%);
[0067] Prepare a 5wt% slu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com