A kind of method that solvent extraction prepares potassium nitrate
A potassium nitrate and extraction technology, applied in the field of solvent extraction to prepare potassium nitrate, can solve the problems of unsatisfactory extraction effect, high separation cost, large consumption of isoamyl alcohol, etc., achieve easy control of extraction and back-extraction operations, simplify extraction and Stripping operation, good extraction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
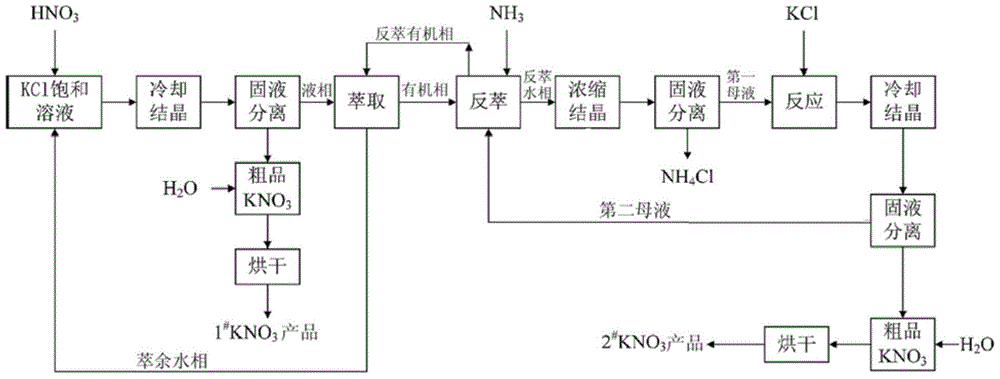
[0028] The process flow of this embodiment is as figure 1 As shown, the steps are as follows:
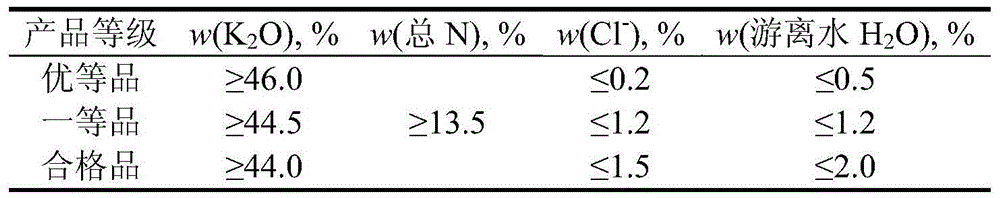
[0029] (1) Add 100g of potassium chloride, 233g of water and the raffinate aqueous phase (at the beginning of the process, the raffinate aqueous phase is water) into the reaction vessel, prepare a saturated potassium chloride solution at a temperature of 50℃, and add it to the reaction vessel 103g nitric acid with a concentration of 68wt% was reacted at 50℃ for 60min under normal pressure and stirring. The resulting reaction solution was naturally cooled to 25℃, filtered, and the resulting filter cake was washed with 20g of water and dried to obtain 1 # 63g of potassium nitrate product, with a yield of 56%;
[0030] (2) Step (1) Filter to obtain 364g filtrate, in which H + The content is 0.30wt%, Cl - The content is 12.89wt%, NO 3 - The content is 8.75% by weight, the extractant is added to the filtrate, the volume ratio of the extractant to the filtrate is 1.5:1, the extraction is perfor...
Embodiment 2
[0041] The process flow of this embodiment is as figure 1 As shown, the steps are as follows:
[0042] (1) Add 100 g of potassium chloride, 218 g of water and the raffinate aqueous phase (at the beginning of the process, the raffinate aqueous phase is water) into the reaction vessel, prepare a saturated potassium chloride solution at a temperature of 60°C, and add it to the reaction vessel 83g of nitric acid with a concentration of 68wt%, reacted at 60°C for 45min under normal pressure and stirring, the resulting reaction liquid was naturally cooled to 30°C, filtered, and the resulting filter cake was washed with 25g of water and dried to obtain 1 # 49g of potassium nitrate product, with a yield of 54%;
[0043] (2) Step (1) filter to obtain 341g filtrate, in which H + The content is 0.26wt%, Cl - The content is 13.54wt%, NO 3 - The content is 7.79wt%, the extractant is added to the filtrate, the volume ratio of the extractant to the filtrate is 1:1, the extraction is carried out at...
Embodiment 3
[0052] The process flow of this embodiment is as figure 1 As shown, the steps are as follows:
[0053] (1) Add 100 g of potassium chloride, 208 g of water and the raffinate aqueous phase (at the beginning of the process, the raffinate aqueous phase is water) into the reaction vessel, prepare a saturated potassium chloride solution at a temperature of 70°C, and add it to the reaction vessel 130g of nitric acid with a concentration of 65% by weight, stirred and reacted at 70°C under normal pressure for 30min, the resulting reaction liquid was naturally cooled to 20°C, filtered, and the resulting filter cake was washed with 15g of water and dried to obtain 1 # 72g of potassium nitrate product, with a yield of 53%;
[0054] (2) Step (1) Filter to obtain 361g filtrate, in which H + The content is 0.37wt%, Cl - The content is 13.22wt%, NO 3 - The content is 11.21wt%, the extractant is added to the filtrate, the volume ratio of the extractant to the filtrate is 3:1, the extraction is carri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com