Purifying and renaturation method for scorpion toxin protein inclusion body and application
A technology of scorpion venom protein and inclusion body, applied in the field of bioengineering, can solve the problems of low yield and high yield, and achieve the effects of improving yield, easy operation and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Construction of recombinant plasmids and bacterial strains.
[0041] (1) pET29a / C His6 -Construction of rAGAP plasmid:
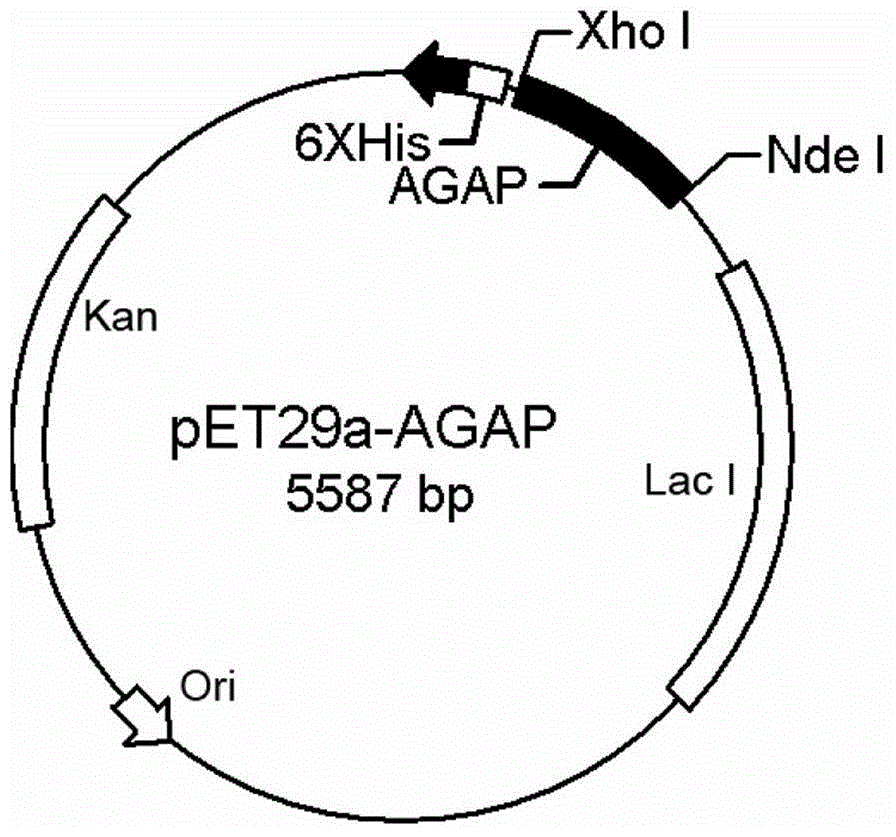
[0042] Entrust Gill Biochemical (Shanghai) Co., Ltd. to synthesize the mature polypeptide sequence of AGAP (the polypeptide sequence is well known to those skilled in the art), and use P1 and P2 primers to amplify the target gene. The primers contain NdeI and XhoI restriction endonuclease recognition sites, and the target gene is cloned into the pET29a plasmid to obtain pET29a / C His6 -rAGAP recombinant plasmid ( figure 1 ). The recombinant plasmid was transformed into E.coliDH5α, and DNA sequencing was carried out after screening in LB medium containing 50 μg / ml kanamycin to identify whether the target gene was correctly introduced into the strain. The strains containing the recombinant plasmids were expanded and cultivated, the recombinant plasmids were extracted, and transformed into E. coliBL21 (DE3) expression strains.
[0043] P1(...
Embodiment 2
[0049] Example 2: Expression and purification of rAGAP.
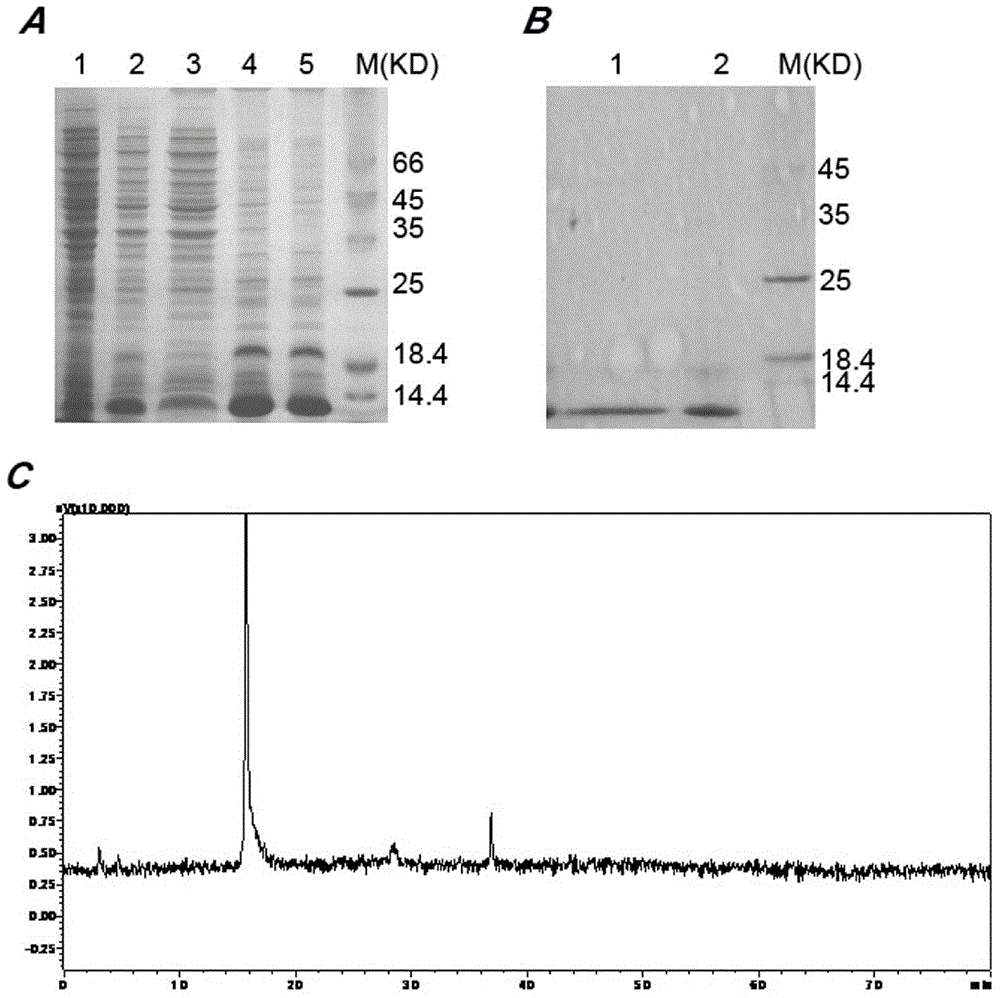
[0050] Pick single clones of the E.coliBL21(DE3) strains containing the above recombinant plasmids and culture them overnight in 50mL LB medium (containing 50mg / mL kanamycin) at 37°C and 220rpm, and take 25mL overnight medium to 1L fresh Cultured in LB medium until logarithmic growth phase. When the absorbance OD 600 When it reaches 0.6-0.7, add 1mmol / LIPTG to induce the expression of the target protein at 37°C for 4h. The bacterial solution was centrifuged, resuspended with 100mL lysate (100mmol / LNaCl, 50mmol / LTris, 2mmol / LEDTA, 1% v / vtritonX-100, pH8.0), and sonicated at 400w for 30min in an ice bath. The cell lysate was centrifuged at 12000rpm for 10min. SDS-PAGE found that almost 90% rAGAP was expressed in the form of inclusion bodies ( figure 2 A). The insoluble matter was resuspended with 50mL eluent (100mmol / LNaCl, 50mmol / LTris, 2mmol / LEDTA, 2mol / LUrea, pH8.0), and then sonicated for 5min. After centrifuga...
Embodiment 3
[0051] Example 3: Renaturation of rAGAP.
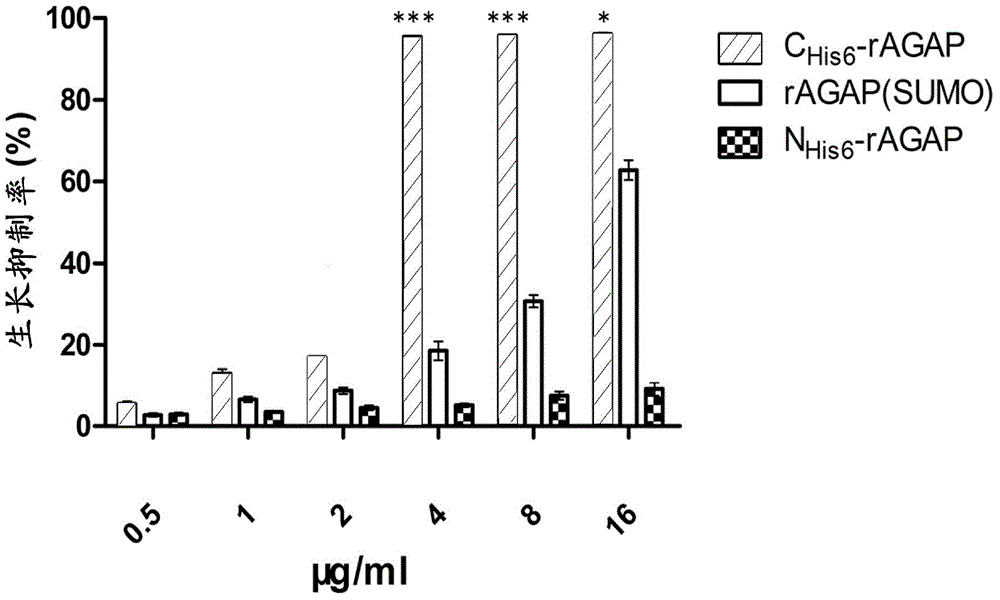
[0052] With the denatured rAGAP obtained in Example 2, use refolding buffer (100mmol / LNa 2 HPO 4 , 50mmol / LTris, pH8.5, 0.5mol / LL-Arg, 2mmol / LEDTA, 1mmol / LGSH, 0.1mmol / LGSSG, 5% v / v glycerol, 0.2% v / vtriton-100) dissolved to a final protein concentration of 0.1 mg / ml, and then incubated at 20°C for 24h. After centrifugal filtration, the supernatant was concentrated 20 times by the Labscale TFF ultrafiltration system, dialyzed with 1×PBS, pH7.4 buffer for 36 hours, and the buffer was changed every 12 hours. After renaturation, 1L medium can obtain 16mg soluble scorpion toxin. The purity of rAGAP is identified by reversed-phase HPLC analysis, and the purity can reach 95% ( figure 2 B,C). C His6- The yield and purity of each step in the rAGAP purification process are shown in Table 1. The key reagents in this embodiment are the concentration of L-Arg and the concentration ratio of GSH-GSSG.
[0053] N His6- Purification and ren...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com