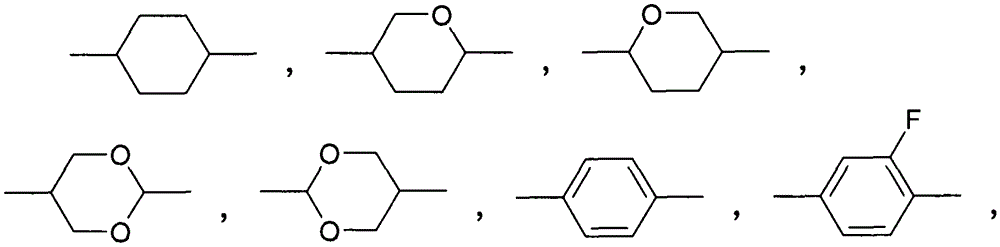
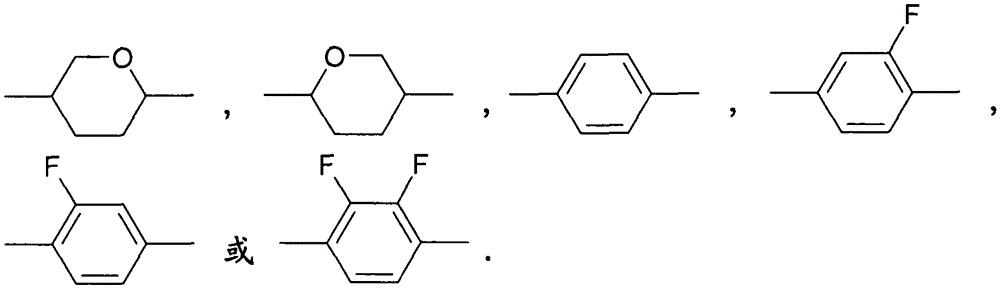
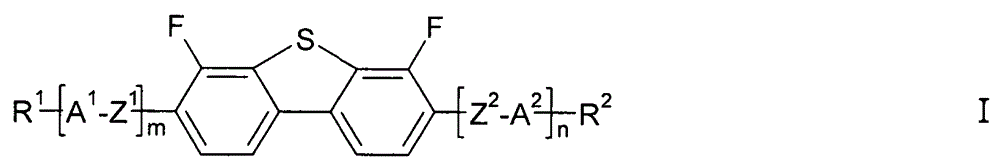4,6-difluoro dibenzothiophene derivates
A compound, -OCF2- technology, applied in 4 fields, can solve the problems of unfinished research and development in the field of liquid crystal materials, and achieve high-definition bright spots, good low temperature and long-term stability, and easy preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1: 3-ethoxy-4,6-difluoro-7-pentyldibenzothiophene
[0153]
[0154] step 1
[0155]
[0156] 0.058 mol of 2-bromo-6-fluorophenol was dissolved in 100 ml of THF and 40 ml of water, and 0.09 mol of potassium carbonate was added. After warming to the boiling point, 0.3 mmol of tris(dibenzylideneacetone) dipalladium (0) and 0.9 mmol of A (bis(1-adamantyl)-n-butylphosphine), and 0.062mol (4-ethoxy-2,3-difluorobenzene base)-dimethoxyborane solution. The mixture was boiled at reflux for another 16 hours, then water and MIB were added, and the mixture was subjected to extractive work-up. The crude product 4'-ethoxy-3,2',3'-trifluorobiphenyl-2-ol was purified by chromatography (eluent: chlorobutane) to give white crystals.
[0157] step 2
[0158]
[0159] 0.022 mol of 4'-ethoxy-3,2',3'-trifluorodiphenyl-2-ol, 0.036 mol of triethylamine and 0.6 mmol of DMAP were dissolved in 50 ml of dichloromethane. 0.03 mol of trifluoromethanesulfonic anhydride was a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com