Indene derivative used as component of liquid crystal medium, preparation method thereof and application thereof
A reaction and compound technology, applied in the preparation of organic compounds, liquid crystal materials, preparation of halogenated hydrocarbons, etc., can solve problems such as low resistivity and voltage retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
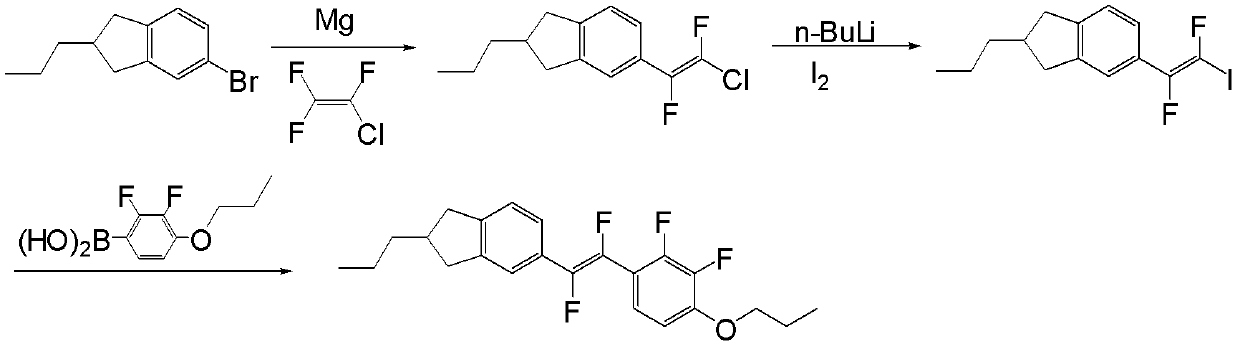
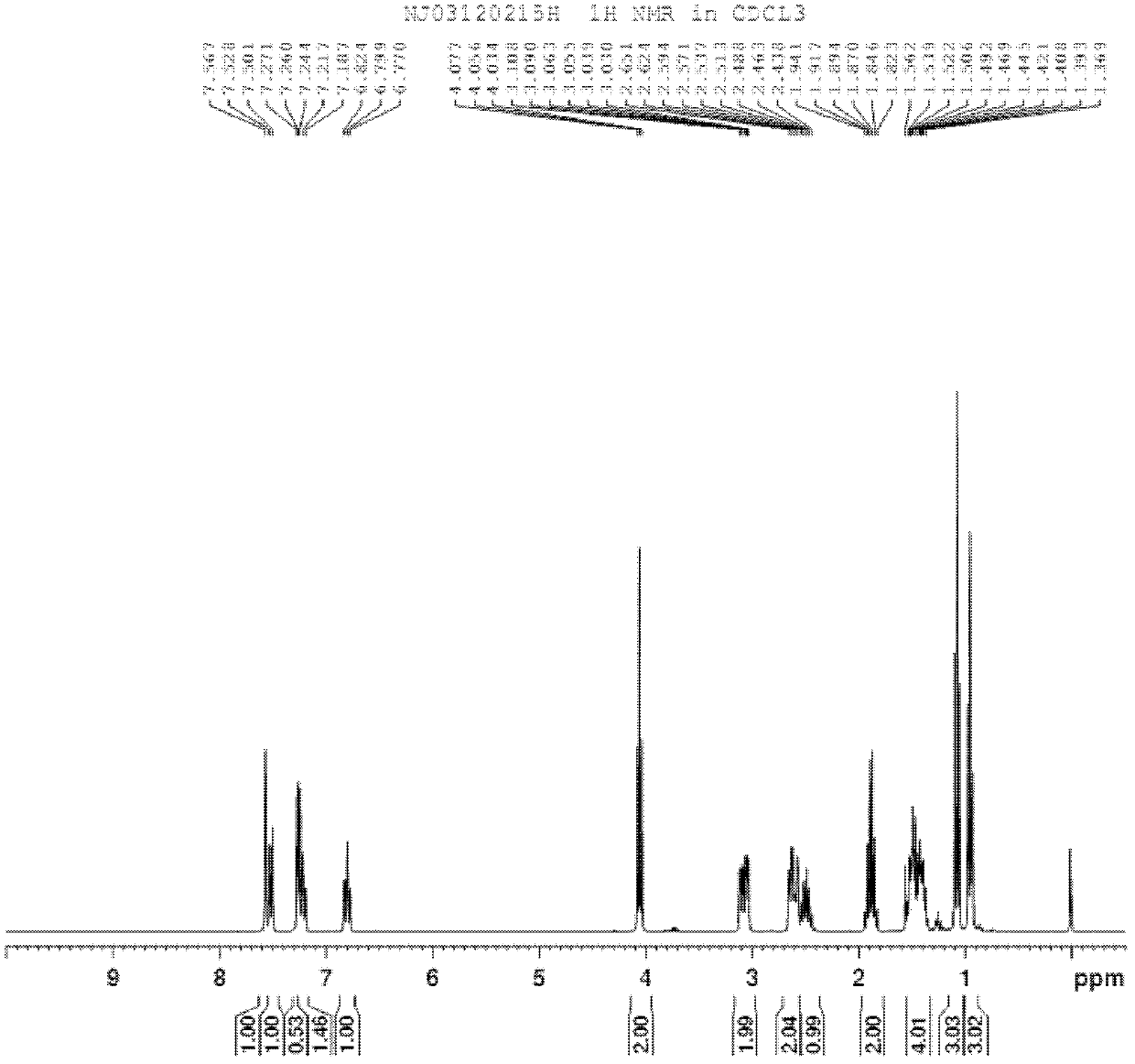
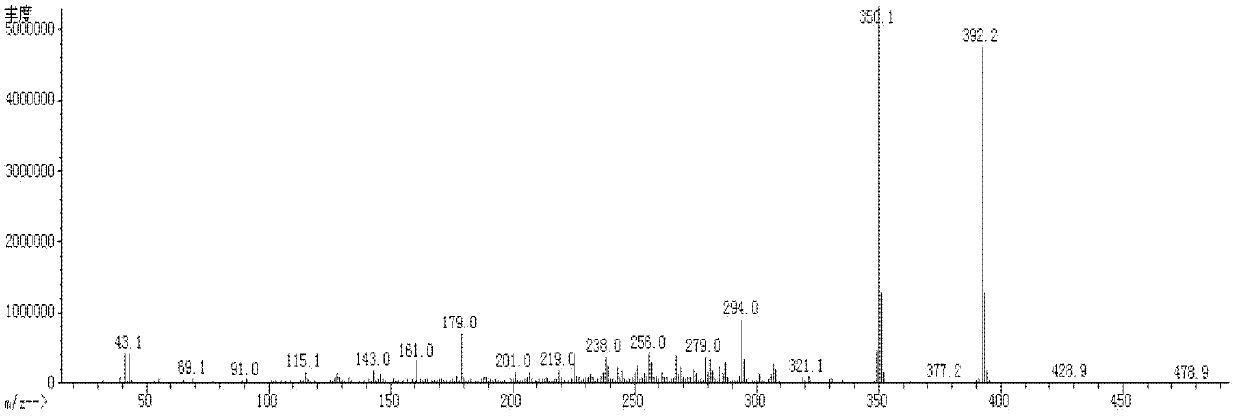
[0068] The synthetic route of preparing compound I-1-1 is in figure 1 Said in, its specific process steps are as follows:
[0069]
[0070] 1) compound of synthetic formula 1
[0071] (Formula 1)
[0072] (Formula 2)
[0073] Add 8g of Mg powder and 0.05g of iodine into a 500mL three-necked flask, and 10mL of tetrahydrofuran (THF), under nitrogen protection. Dissolve 45g of the compound of formula 2 in 200mL of tetrahydrofuran solution, slowly add the tetrahydrofuran solution of the compound of formula 2 to the above reaction system, after the reaction is triggered, continue to drop the remaining tetrahydrofuran solution of the compound of formula 2, and drop , keep the system slightly boiling and reflux for 3h, and cool to room temperature. 40g of chlorotrifluoroethylene was slowly passed into the cooled reaction system, and stirred at room temperature for 12h.
[0074] The reaction was quenched with dilute hydrochloric acid, extracted with ethyl acetate, the orga...
Embodiment 2
[0093] The synthetic route of preparing compound I-2-1 is in Figure 4 Said in, its specific process steps are as follows:
[0094]
[0095] 1) The compound of formula 3 was prepared by steps 1)-2) in Example 1.
[0096]2) compound of synthetic formula 6
[0097] (Formula 6)
[0098] (Formula 7)
[0099] (Formula 8)
[0100] (Formula 9)
[0101] In a 250mL round bottom flask, add 9.3g of the compound of formula 7, 6.4g of the compound of formula 8, 100mL of ethylene glycol dimethyl ether, 35mL of potassium carbonate aqueous solution (2mol L -1 ), 0.5g tetrabutylammonium bromide, 0.4g Pd(PPh 3 ) 4 , nitrogen protection, reflux for 5h. The reaction was quenched with water, extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, the solvent was spin-dried, petroleum ether was used as the mobile phase for column chromatography, and the eluent was concentrated to obtain 7.6 g of a colorless liquid to obtain a compound of formula 9...
Embodiment 3
[0112] The synthetic route of preparing compound I-3-1 is in Figure 7 Said in, its specific process steps are as follows:
[0113]
[0114] 1) The compound of formula 3 was prepared by using the process steps 1)-2) in Example 1.
[0115] 2) compound of synthetic formula 10
[0116] (Formula 10)
[0117] (Formula 11)
[0118] In a 250mL round bottom flask, add 6g of the compound of formula 11, 8.5g p-phenylbromoiodide, dissolve in 100mL ethylene glycol dimethyl ether, and dissolve in 30mL potassium carbonate aqueous solution (2mol L -1 ), 0.5g tetrabutylammonium bromide, 0.4g Pd(PPh 3 ) 4 , nitrogen protection, reflux reaction for 5h. The reaction was quenched with water, extracted with ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, the solvent was spin-dried, petroleum ether was used as the mobile phase for column chromatography, and the eluent was concentrated to obtain 8.5 g of a white solid to obtain the compound of formula 10.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com