A kind of synthetic method of the moCys fragment of marine natural product apratoxin E
A technology of natural products and synthetic methods, applied in the preparation of sulfides, organic chemistry, etc., can solve the problems of unsuitability for industrial production and high price, and achieve the effects of high total yield, easy reaction conditions, and good product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
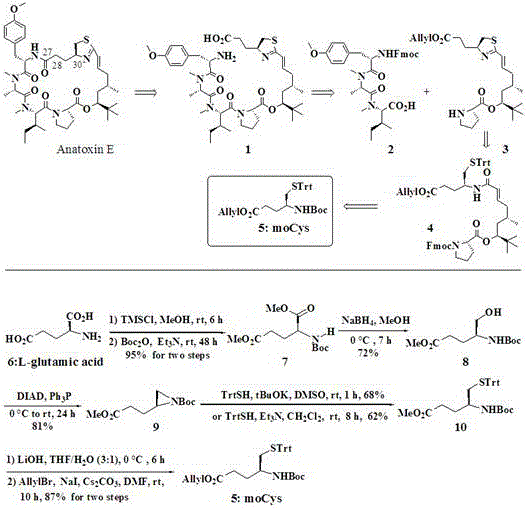
[0017] Example 1: Synthesis of N-Boc L-glutamate
[0018] 7.4 g of L-glutamic acid (6, 50.0 mmol) and 100 ml of methanol were added to the reaction flask, 32 ml of trimethylchlorosilane (TMSCl, 250.0 mmol) was added dropwise under zero degree conditions, and the mixture was stirred at room temperature for 6 hours. TLC detected the ester The chemical reaction is over. Add 49 ml of triethylamine (Et 3 N, 350.0 mmol) and 13.2 g Boc 2 O (60.0 mmol), stirring at room temperature for 48 hours. The ethanol was recovered by distillation under reduced pressure, 200 ml of ethyl acetate and 100 ml of water were added, and the layers were separated after shaking. The aqueous phase was extracted with ethyl acetate (100 mL x 3). The organic phases were combined, dried over anhydrous sodium sulfate, filtered, and concentrated. Purification by column chromatography yielded 13.1 g of N-Boc L-glutamate (7). The total yield of 2 steps is 95%. Optical rotation [α] D 28 +12.7° ( c = 1.8, CHCl 3 )...
Embodiment 2
[0019] Example 2: Synthesis of N-Boc acridinium ester
[0020] 2.8 g of N-Boc L-glutamate (7, 10.0 mmol) and 20 ml of methanol were added to the reaction flask, and 0.5 g of sodium borohydride (NaBH 4 , 12.0 mmol) and stirring for 7 hours. TLC detects the end of the reaction and adds 20 ml of water to quench it. After no bubbles are generated, the anhydrous methanol is evaporated to dryness with a rotary evaporator, extracted with ethyl acetate (50 mL x 3), the organic phases are combined, and dried with anhydrous sodium sulfate. Filter, concentrate, and purify by column chromatography to obtain 1.8 g of o-amino alcohol (8) with a yield of 72%. Take 1.1 g of o-amino alcohol (7, 4.3 mmol) and dissolve in 10 ml of tetrahydrofuran. Add 1.0 g of DIAD (5.1 mmol) and 1.5 g of triphenylphosphine (5.5 mmol) to the reaction system under zero degree conditions, and stir at room temperature for 24 hours. TLC detects the end of the reaction, adds 20 mL of water to quench, extracts with eth...
Embodiment 3
[0021] Example 3: Synthesis of moCys fragment of marine natural product apratoxin E
[0022] 1.15 g of N-Boc acridinium ester (9, 5.0 mmol) was dissolved in 10 ml of DMSO, and 1.41 g of trityl mercaptan (98%, 5.0 mmol) and 0.56 g of potassium tert-butoxide (5.0 mmol), stirring at room temperature for 1 hour. TLC detects the end of the reaction, adds 20 mL of saturated aqueous ammonium chloride to quench, extracts with ethyl acetate (50 mL x 3), combines the organic phases, dried over anhydrous sodium sulfate, filtered, concentrated, and purified by column chromatography to obtain the ring-opened product (9, the yield is 81%). Take 0.51 g of the ring-opened product (9, 1.0 mmol), dissolve in 3 ml of tetrahydrofuran and 1 ml of water, add 3 mmol of LiOH, and stir for 6 hours at zero degrees. TLC detects the end of the reaction. Adjust the pH to 6, extract with ethyl acetate (20 mL x 3), combine the organic phases, dry with anhydrous sodium sulfate, filter, and concentrate, then d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com