A kind of tilmicosin drug inclusion compound and its preparation and application
A technology of tilmicosin and clathrate, which is applied in the field of veterinary biological products, can solve the problems of complex preparation process, many raw materials for coating liquid preparation, etc., achieve simple preparation process, low production cost, and reduce raw materials The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of tilmicosin drug inclusion compound
[0058] 1 Preparation of core particles loaded with tilmicosin
[0059] (1) The former powder of tilmicosin that takes 10g is put into 1000ml beaker, adds the 95% ethanol solution of 200ml, stirs until tilmicosin dissolves completely, the beaker that mixed solution is housed is placed on the magnetic stirrer, 25 ℃, 600r / min stirring constantly, slowly add 200ml of silica sol, the solution becomes turbid.
[0060] (2) After continuous stirring for 2 hours, the mixed solution was divided into 15ml centrifuge tubes, each tube was filled with 10ml solution, and centrifuged at 25°C and 8000r / min for 20min. Separate the supernatant from the precipitate.
[0061] (3) Place the precipitate in a constant temperature drying oven at 60°C, and after drying for 4 hours, take out the precipitate in all the centrifuge tubes and mix them together, and grind them through a 100-mesh sieve.
[0062] 2 Preparation of c...
Embodiment 2
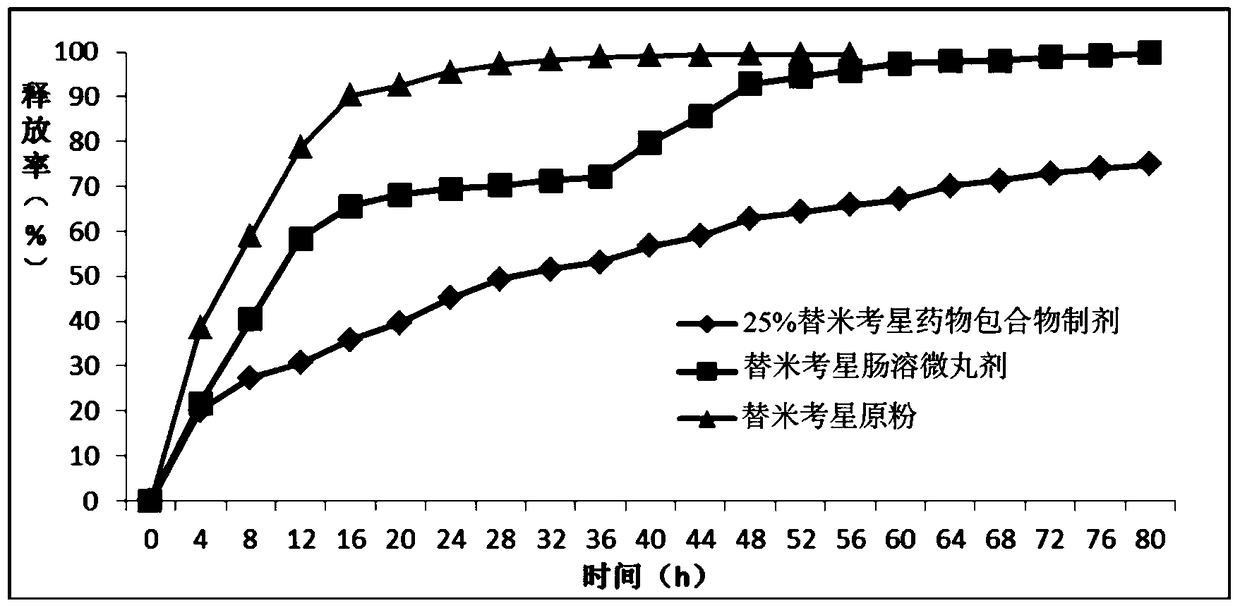
[0069] Embodiment 2: The release performance test of tilmicosin drug inclusion compound
[0070] 1 Preparation of buffer
[0071] (1) Weigh 32.8g of sodium phosphate, add a small amount of purified water and stir to fully dissolve the sodium phosphate, add purified water to dilute to 1000ml, shake and shake to obtain a sodium phosphate solution with a concentration of 0.2mol / l.
[0072] (2) Measure 250ml of the above-mentioned sodium phosphate solution with a graduated cylinder, mix it evenly with a hydrochloric acid solution of 0.1mol / l with a concentration of 750ml, adjust the pH value to 6.86 with 2mol / l hydrochloric acid, and finally obtain a phosphate with a pH value of 6.86 buffer.
[0073] 2 Determination of sustained release rate
[0074] Accurately weigh the tilmicosin drug inclusion complex preparation 0.4g, tilmicosin former powder 0.12g and tilmicosin enteric-coated pellets 0.5g (ground into powder) prepared by embodiment 1 with a drug loading of 25%. shape). P...
Embodiment 3
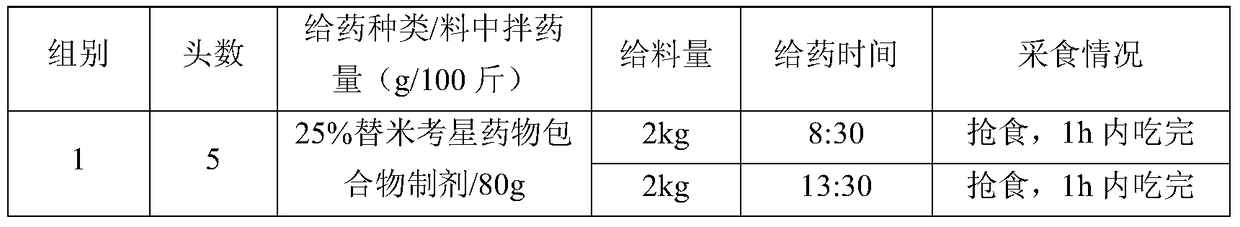
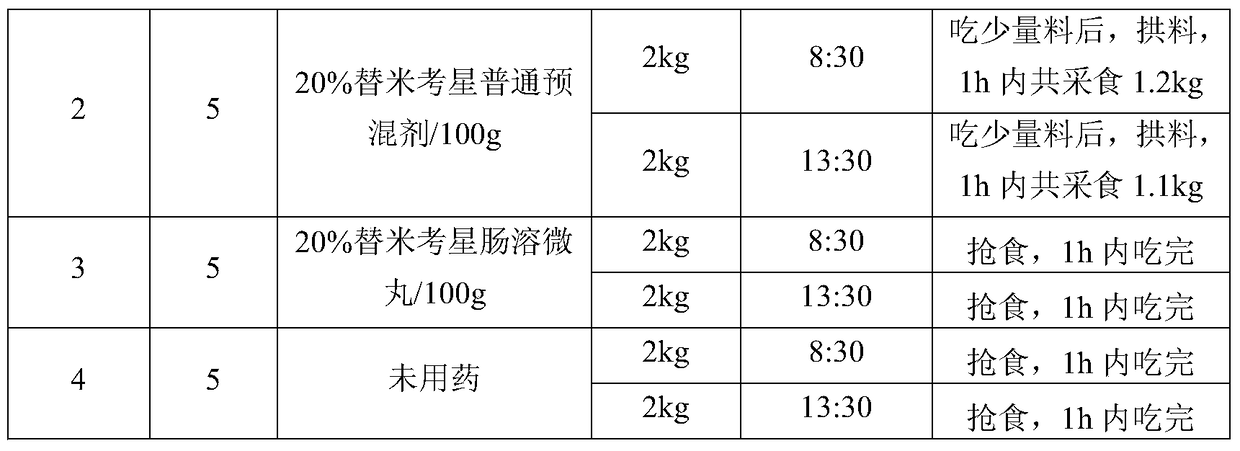
[0077] Embodiment 3: the palatability test of tilmicosin drug inclusion compound
[0078] 1 Test drug and feed
[0079] The tilmicosin drug inclusion compound preparation with a drug loading of 25% prepared in Example 1.
[0080] Tilmicosin common premix, content 20%, batch number: 20130502, Henan Hemu Animal Pharmaceutical Co., Ltd.
[0081] Tilmicosin enteric-coated pellets, content 20%, batch number: W130711 Hubei Longxiang Pharmaceutical Co., Ltd.
[0082] Common feed for pig farms, Hefeng Feed.
[0083] 2 Experimental animals and groups
[0084] The test pigs were self-raised by the farm. Select 20 pigs with a body weight of 20-30kg and divide them into 4 groups with 5 pigs in each group. The first group is the test group, the second group is the 20% tilmicosin common premix control group, and the third group is the replacement group. Micoxin enteric-coated pellets, the fourth group is the normal feeding group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com