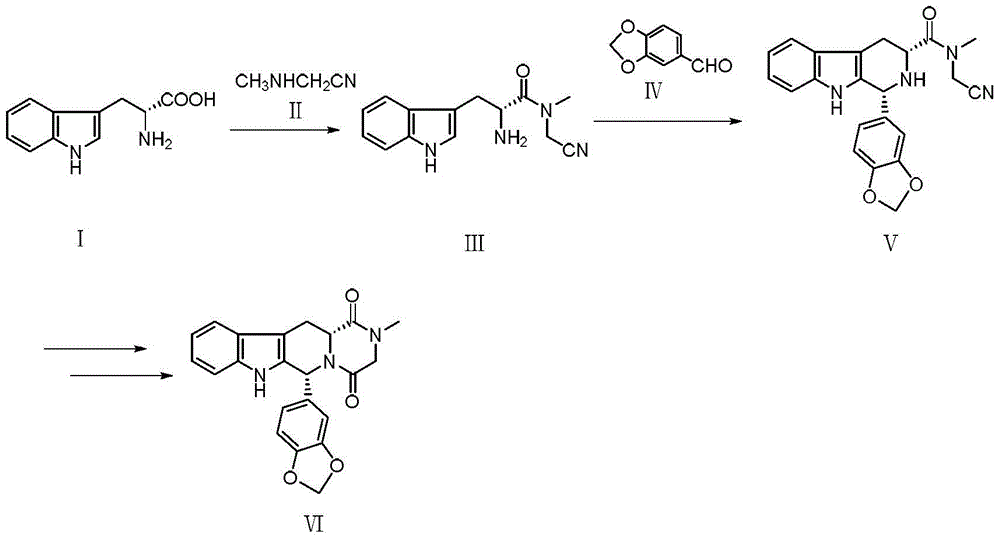
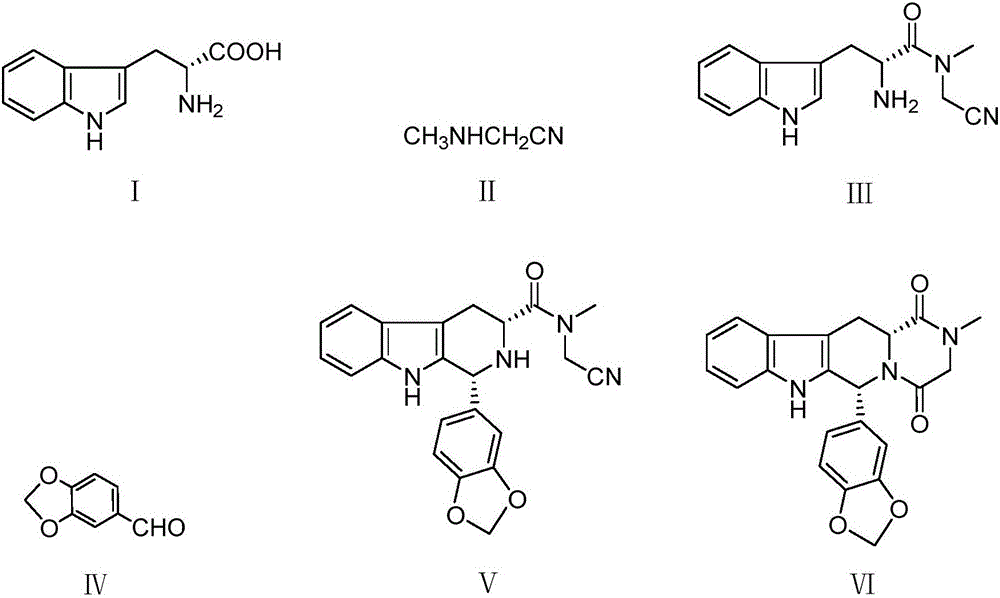
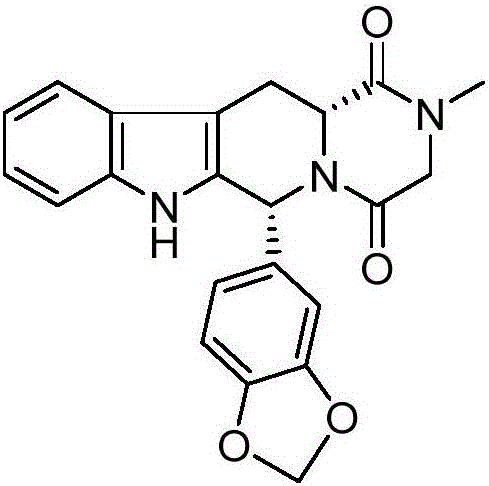
A kind of tadalafil synthetic method
A technology of tadalafil and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of inflammability, high toxicity, environmental pollution and the like, and achieve the effects of simple reaction method, convenient operation and serious environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 200ml of tetrahydrofuran, 20g of D-tryptophan, 7.7g of methylaminoacetonitrile, 38.2g of EDC·HCl and 1g of 4-dimethylaminopyridine were added to a 1L three-necked flask, and the reaction was stirred at room temperature for 5 hours. After the reaction is complete, add 300ml of ethyl acetate and 300ml of water, stir and separate the layers, add 200ml of saturated sodium chloride to the ethyl acetate layer, stir and wash, let stand and separate the layers, and distill the ethyl acetate layer under reduced pressure at 30°C to a volume of about 200ml , adding 700ml of petroleum ether to crystallize, filter, and vacuum-dry at 40°C to obtain 22.25g of compound III. HPLC purity 98%, yield 91%. 1 H NMR (500MHz, DMSO), δ: 3.27-3.38(m, 2H), 3.09(s, 3H), 3.91(s, 1H), 4.16(t, 1H), 6.98(t, 1H), 7.07(t , 1H), 7.25(d, J=2Hz, 1H), 7.35(d, J=8Hz, 1H), 7.50(d, J=8Hz, 1H), 8.76(s, 2H), 11.19(s, 1H) ; MS(m / z): 257.1[M+1] + .
Embodiment 2
[0034] According to the operation of Example 1, the influence of solvent and reaction temperature on the yield of compound III was investigated, and the results are shown in Table 1.
[0035] Table 1: Effect of solvent and reaction temperature on the yield of compound Ⅲ
[0036]
[0037]
Embodiment 3
[0039] Add 200ml of isopropanol, 20g of compound III and 12.3g of piperonal into a 250ml three-necked flask, heat to reflux, and stir to react for 20 hours. After the reaction was complete, the temperature was lowered to room temperature and stirred for one hour. After filtering and rinsing with ice isopropanol, 27.6 g of compound V was obtained after vacuum drying. (HPLC purity 98.5%, yield 94%. 1 H NMR (500MHz, DMSO), δ: 3.32(d, J=8Hz, 2H), 3.21(s, 3H), 3.93(s, 2H), 4.66(m, 1H), 5.91(s, 1H), 6.07 (s, 2H), 7.01-7.12(m, 5H), 7.31(d, J=8Hz, 1H), 7.54(d, J=8Hz, 1H), 10.37(s, 1H), 10.79(s, 1H) ; MS(m / z): 389.1[M+1] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com