Application of water-soluble porphyrin in preparing photodynamic therapy medicine
A photodynamic therapy and water-soluble technology, which is applied in the application field of water-soluble porphyrin in the preparation of photodynamic therapy drugs, can solve the problems of high price and unsuitability for widespread popularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
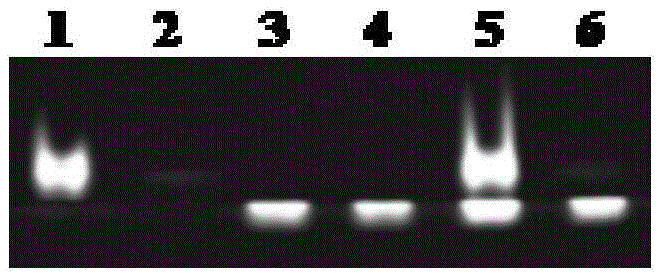
[0028] (1) G-quadruplex photocleavage.
[0029] (A) Sample preparation under simulated physiological conditions: 5 μL of 100 μM G-quadruplex (Hum51, ordered by Shenggong), 8 μL of 250 mM Tris-HCl were added to the four centrifuge tubes numbered 1, 2, 3 and 4 respectively (pH=7.4) buffer solution, 10 μL of 1M KCl solution, ultrapure water, so that the concentration of Hum51 is 5 μM, the concentration of Tris-HCl buffer solution is 20 mM, and the concentration of KCl is 100 mM. Shake and shake the centrifuge tube well, centrifuge the solution to the bottom, place it in a PCR-type gene amplification thermocycler at 95°C for 5 minutes, and cool down.
[0030] (B) Contrast between light and dark conditions:
[0031] Add 40 μL of ultrapure water into the centrifuge tube numbered 1, shake, shake well, centrifuge and place in the dark at 25°C for 2 hours;
[0032] Add 40 μL of ultrapure water into the centrifuge tube numbered 2, oscillate, shake well, and centrifuge, then irradiate ...
Embodiment 2
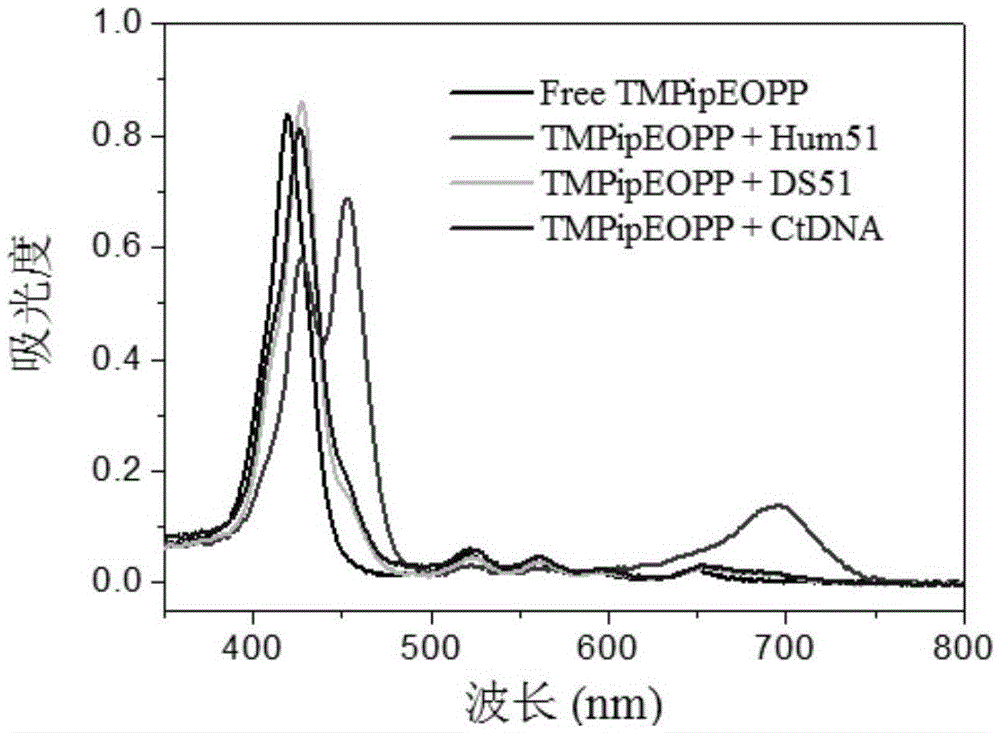
[0057] A kind of preparation of water-soluble porphyrin (I):
[0058] The chemical name of the water-soluble porphyrin (I) is: 5,10,15,20-tetrakis{4-[2-(1-methyl-1-piperidine)ethoxy]phenyl}porphyrin tetraiodide. (referred to as TMPipEOPP)
[0059] (The preparation of this embodiment is to enable those skilled in the art to understand the present invention better, but does not make any limitation to the present invention, the water-soluble porphyrin (I) that other synthetic methods obtain, all can be used in the present invention )
[0060] (1) Synthesis of four (p-hydroxyporphyrin) (II)
[0061] Add 100mM p-hydroxybenzaldehyde and 120mL propionic acid into a four-neck flask, stir, add pyrrole 100mM dropwise under reflux (127°C-132°C), drop it in 15min, and reflux for 120min. Pour it into a beaker while it was hot, stir and cool to room temperature, filter with suction, wash the filter cake with propionic acid, and dry in vacuo to obtain a blue-purple crude product, which wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com