A kind of double-arm benzoic acid organic rare earth high-efficiency light-emitting material and preparation method thereof
A benzoic acid, rare earth luminescence technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as the inability to meet application requirements, and achieve the effect of increasing fluorescence intensity and long fluorescence life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Tb 2 (BCMT) 2 (H 2 O) 4
[0052] Weigh 0.01mol of 1,4-terephthalic acid diglycolate and add it to 40ml of a mixed solvent of ethanol and N,N-dimethylformamide (DMF) (the volume ratio of ethanol to DMF is 1:6 ), fully stirred at 25°C to form a uniform solution 1 (stirring speed was 20r / min). 0.01mol of TbCl 3 After adding to 8ml of ethanol and fully stirring at 25°C until transparent, it was added to solution 1. NH with stirring 3 ·H 2 O adjusted the pH of the mixed solution to be 7, and reacted at 40°C for 60 minutes to obtain solution 2. The solution 2 was heated to 70°C, reacted for 4 hours under the stirring condition of 25r / min, the reaction solution was cooled to room temperature, rotary evaporated, suction filtered, and the product was washed with deionized water and ethanol for 5 times to obtain a sample.
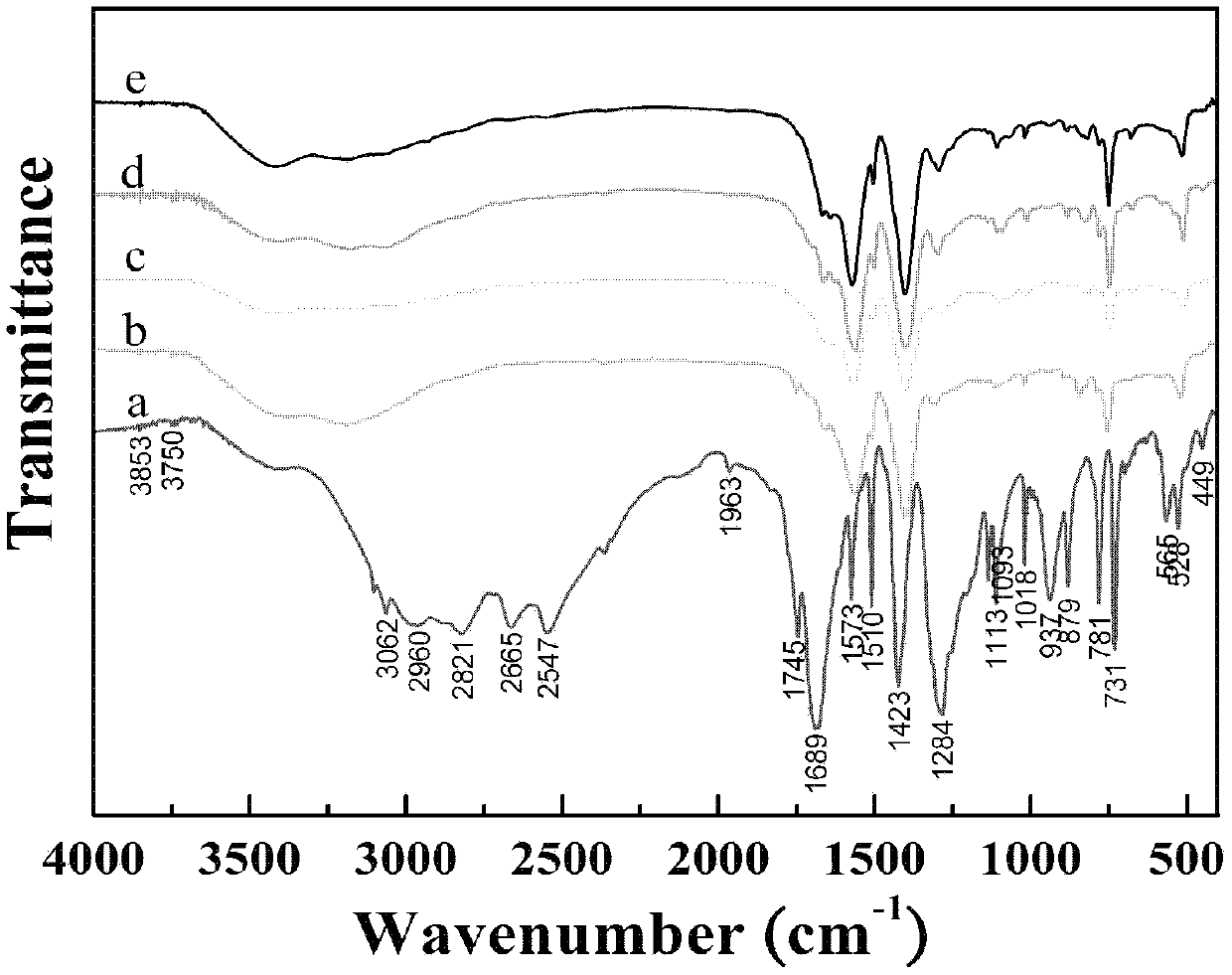
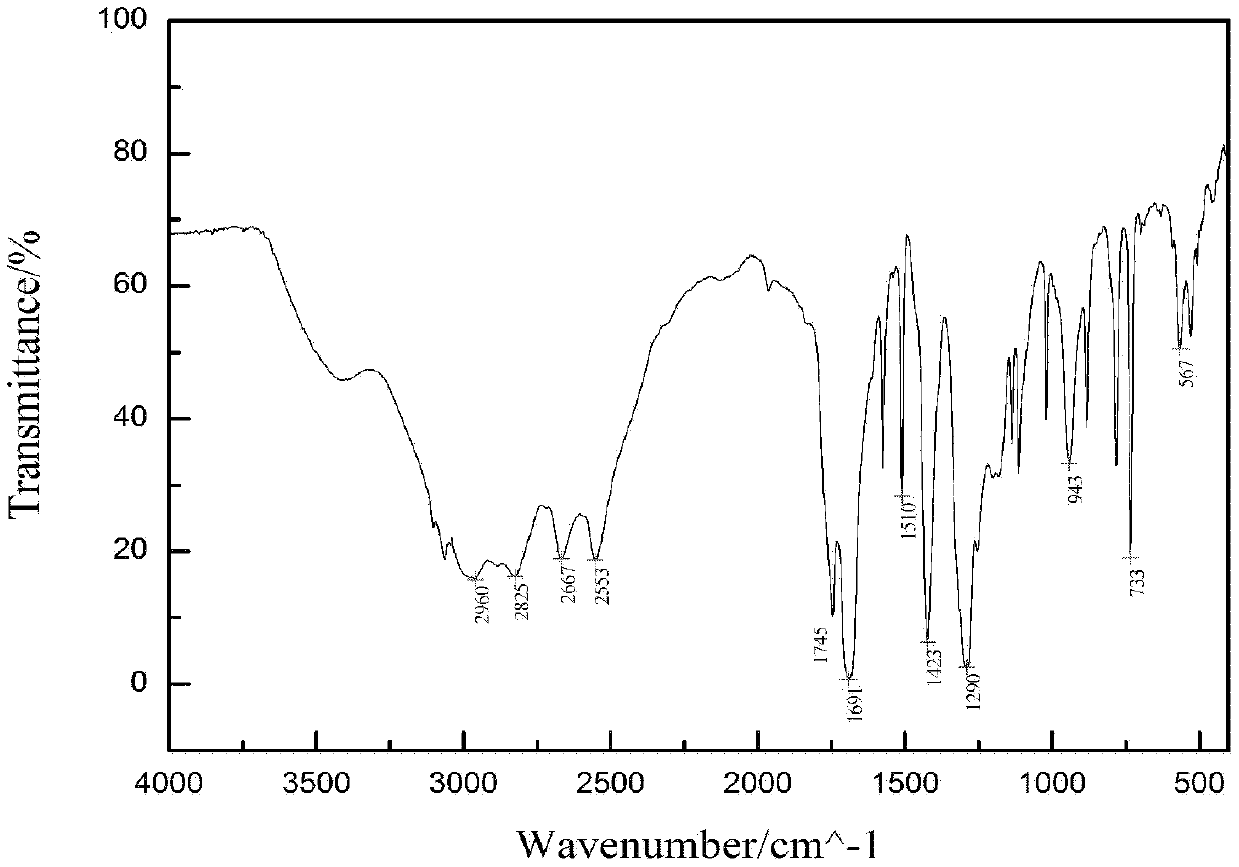
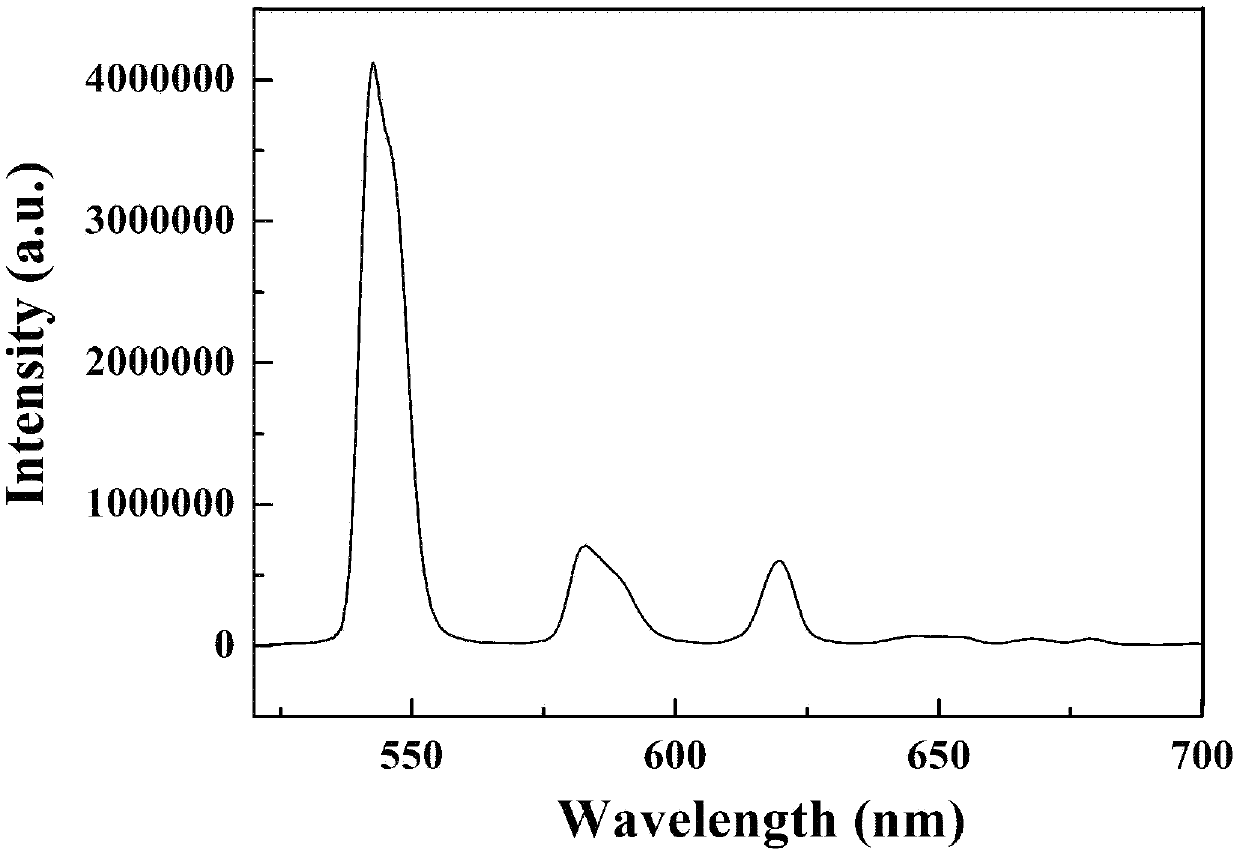
[0053] Such as image 3 and 4 As shown, the fluorescence intensity of the prepared rare earth terbium complex of 1,4-terephthalic acid d...
Embodiment 2
[0054] Example 2: Eu 2 (BCMT) 2 (1,10-Phenanthroline) 2
[0055] Weigh 0.01mol of 1,4-terephthalic acid diglycolate and add it to 60ml of a mixed solvent of ethanol and N,N-dimethylformamide (DMF) (the volume ratio of ethanol to DMF is 6:1 ), fully stirred at 70°C to form a uniform solution 1 (stirring speed is 80r / min). 0.01mol of EuCl 3 After adding to 12ml of ethanol and stirring well at 25°C until transparent, it was added to solution 1. NH with stirring 3 ·H 2 O adjusted the pH value of the mixed solution to be equal to 11, and obtained solution 2 after reacting at 70°C for 30 minutes. Weigh 0.01mol of 1,10-phenanthroline and add it to 8ml of ethanol, stir well at 30°C until it is transparent, then add it to solution 2, react at 60°C and 25r / min for 1 hour, The reaction solution was cooled to room temperature, rotary evaporated, suction filtered, and the product was washed with deionized water and ethanol for 5 times to obtain a sample.
[0056] Such as Figure ...
Embodiment 3
[0057] Example 3: Eu 2 (BCMT) 2 (2-Thienoyltrifluoroacetone) 2
[0058] Weigh 0.01mol of 1,4-terephthalic acid diglycolic acid ester and add it to the mixed solvent of 50ml of ethanol and N,N-dimethylformamide (DMF) (the volume ratio of ethanol and DMF is 1:1 ), fully stirred at 50°C to form a uniform solution 1 (stirring speed was 60r / min). 0.01mol of Eu(NO 3 ) 3 After adding to 10 ml of ethanol and stirring well at 25°C until transparent, it was added to solution 1. NH with stirring 3 ·H 2 O adjusted the pH value of the mixed solution to be equal to 8, and obtained solution 2 after reacting at 50° C. for 45 minutes. Weigh 0.01mol of 2-thienoyltrifluoroacetone and add it to 10ml of ethanol at 50°C until fully stirred until transparent, then add it to solution 2, react at 65°C and 30r / min for 2.5 hours, then react The solution was cooled to room temperature, rotary evaporated, suction filtered, and the product was washed 5 times with deionized water, ethanol and other...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com