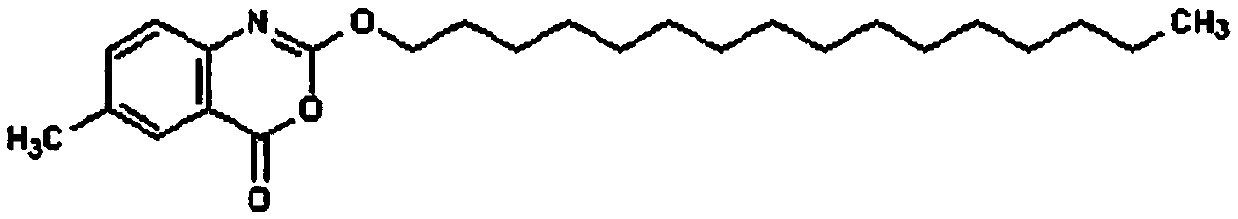
Cetilistat tablets and preparation method thereof
A kind of Lisi, a certain amount of technology, applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of west for Li Sita tablet
[0023] Prescription composition:
[0024] name
weight ratio / %
cetiristat
10
PEG4000
5
2 --> PEG6000
5
Mannitol
35
Microcrystalline Cellulose PH101
34
Hypromellose
5
Low-substituted hydroxypropyl cellulose
5
Magnesium stearate
1
[0025] Preparation Process:
[0026] (1) Dissolve cetiristat in an appropriate amount of absolute ethanol, put PEG4000 and PEG6000 in a 50°C water bath to completely melt, add cetiristat ethanol solution and stir for 1 hour, and remove ethanol by rotary evaporation or drying under reduced pressure , transferred to -20°C for solidification for 12 hours, taken out and placed in a vacuum desiccator to balance for 24 hours, ground into fine powder, and passed through an 80-mesh sieve to obtain a solid dispersion of cetiristat;
[0027] (2) Weigh a certain amount of cetiristat solid d...
Embodiment 2
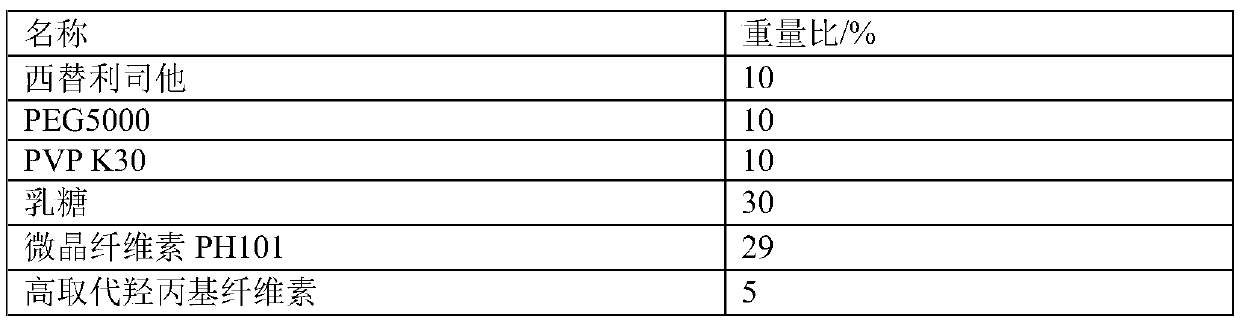
[0028] Embodiment 2: the preparation of west for Li Sita tablet
[0029] Prescription composition:
[0030]
[0031]
[0032] Preparation Process:
[0033] (1) Dissolve cetilistat in an appropriate amount of absolute ethanol, put PEG5000 and PVPK30 in a 50°C water bath to completely melt, add cetilistat ethanol solution and stir for 2 hours, then remove ethanol by rotary evaporation or drying under reduced pressure , transferred to -20°C for solidification for 12 hours, taken out and placed in a vacuum desiccator to balance for 24 hours, ground into fine powder, and passed through an 80-mesh sieve to obtain a solid dispersion of cetiristat;
[0034] (2) Weigh a certain amount of cetiristat solid dispersion and lactose, microcrystalline cellulose PH101, high-substituted hydroxypropyl cellulose and low-substituted hydroxypropyl cellulose, and adopt 20% ethanol aqueous solution to granulate, 24 mesh Granulate, dry at 50°C to 1-3%, granulate with 24 mesh, add stearic acid ...
Embodiment 3
[0035] Embodiment 3: the preparation of west for Li Sita tablet
[0036] Prescription composition:
[0037] name
weight ratio / %
cetiristat
10
PEG4500
12
PVP K90
8
Sorbitol
30
Microcrystalline Cellulose PH101
29
3 --> Hypromellose
5
Crospovidone
5
Magnesium stearate
1
[0038] Preparation Process:
[0039] (1) Dissolve cetilistat in an appropriate amount of absolute ethanol, put PEG4500 and PVPK90 in a 50°C water bath to completely melt, add cetilistat ethanol solution and stir for 1.5h, then remove by rotary evaporation or drying under reduced pressure Ethanol, transferred to -20°C for solidification for 12 hours, taken out and placed in a vacuum desiccator to balance for 24 hours, ground finely, and passed through a 80-mesh sieve to obtain a solid dispersion of cetiristat;
[0040] (2) Take a certain amount of cetiristat solid dispersion and sorbitol, microcrystalline ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap