A kind of contraceptive DNA vaccine and preparation method thereof
A technology of DNA vaccines and nucleotide sequences, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, antibody medical ingredients, etc., and can solve problems such as failure, irreversibility, and harmfulness to the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of the DNA vaccine of the present invention
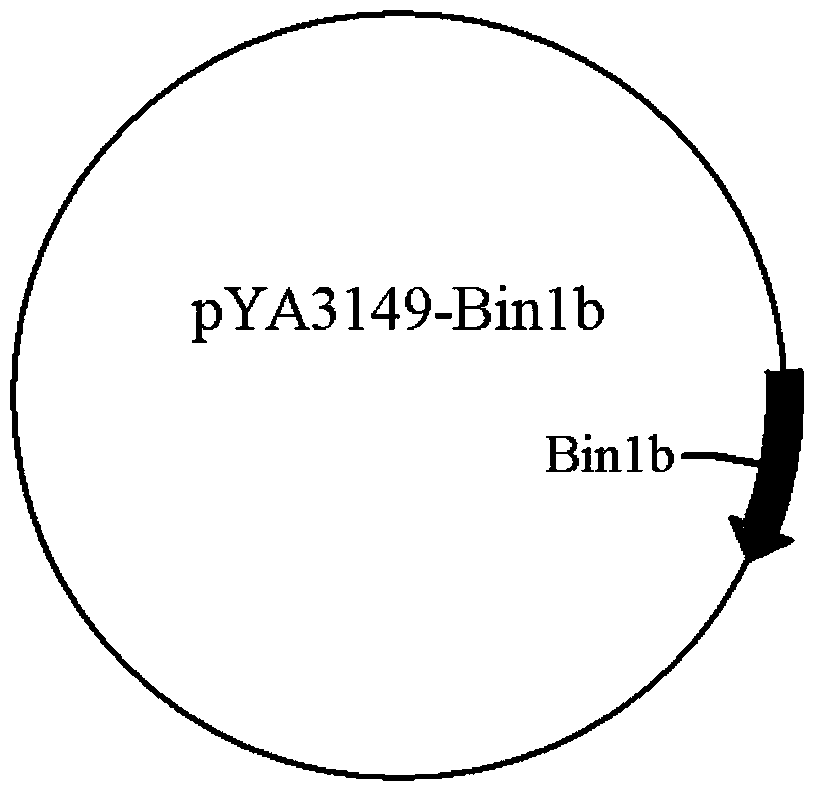
[0051] PCR amplification was performed with pYA3149-Bin1b as the template to obtain the target fragment with a size of 337bp, and the agarose gel electrophoresis detection was consistent with the expected size ( Image 6 ). Among them, the PCR amplification procedure is:
[0052] Pre-denaturation at 94°C for 5min;
[0053] Denaturation at 94°C for 30s, renaturation at 55°C for 30s, extension at 72°C for 60s, cycle 30 times;
[0054] Extend at 72°C for 10 min and store at 4°C.
[0055] The PCR amplification system is:
[0056]
[0057] add ddH 2 O to 50ul, mix well.
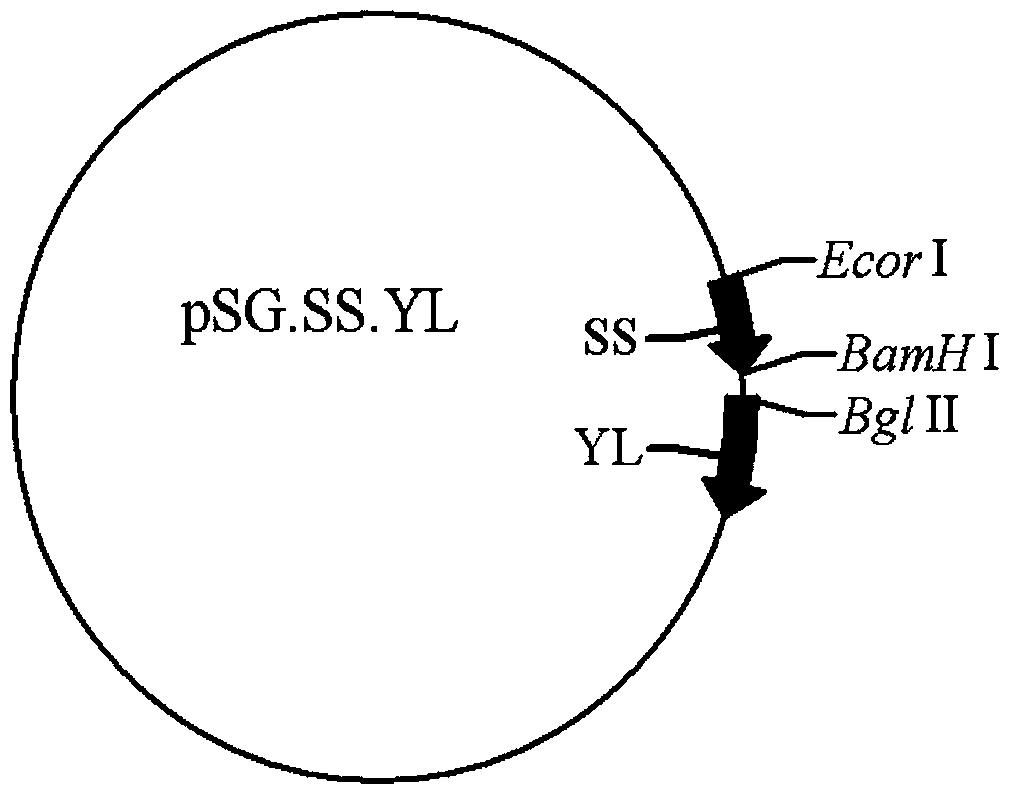
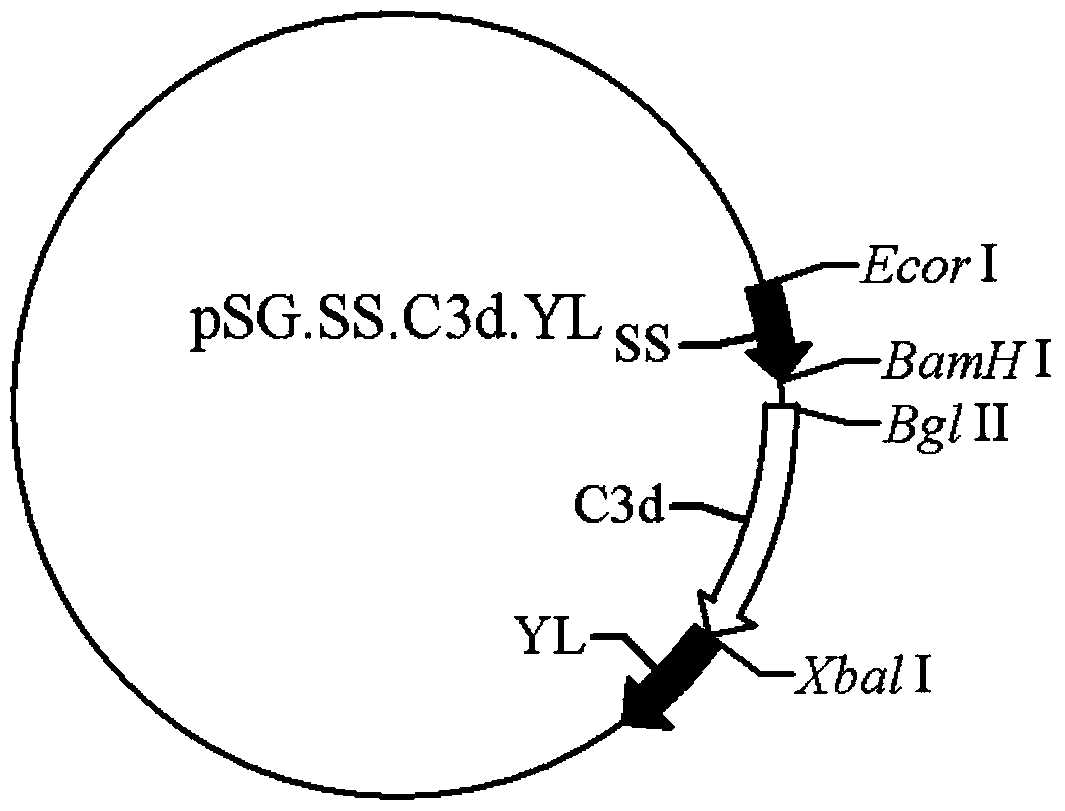
[0058] The PCR products and pSG.SS.C3d3.YL and pSG.SS.YL were digested with BglII, respectively, and were recovered with a PCR product purification kit. The recovered products were digested with BamHI, and digested with 0.8% agarose gel electrophoresis. After the product is obtained, the gel containing the target fragment and the...
Embodiment 2
[0060] Example 2: Validation of recombinant plasmids
[0061] The extracted plasmids pSG.SS.C3d3.Bin1b and pSG.SS.YL.Bin1b were identified by double digestion with BglII and BamHI, and the PCR results were used as control. 0.8% agarose gel electrophoresis digestion results showed that the band near 337bp was the target fragment after digestion, and the sequencing results also showed that this fragment was the target gene, indicating that pSG.SS.C3d3.YL.Bin1b and pSG. SS.YL.Bin1b was built successfully.
Embodiment 3
[0062] Example 3: Indirect immunofluorescence detection of recombinant plasmid Bin1b expression
[0063] Human embryonic kidney HEK293 cells were transfected with pSG.SS.C3d3.YL.Bin1b and pSG.SS.YL.Bin1b, and transfected using Lipofectamine transfection kit.
[0064] After 48 hours of transfection, the coverslips were taken out and fixed in ice acetone for 5 minutes. Enzyme repair and antigen blocking were performed according to the SP9000 kit. Bin1b antibody (1:400) was added and incubated for 40 minutes. Goat IgG (1:500 dilution) was incubated at room temperature for 30 minutes, and the results were observed by fluorescence microscope.
[0065] Compared with the negative immunofluorescence results of pSG.SS.C3d3.YL empty vector, the expression results of pSG.SS.YL.Bin1b and pSG.SS.C3d3.YL.Bin1b showed that a large number of target proteins were expressed in the cytoplasm and nucleus , indicating that the DNA vaccine of the present invention successfully expresses Bin1b in e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com