Method for preparing aldehyde through olefin hydroformylation
A technology of olefin hydroformyl and hydroformyl, which is applied in chemical instruments and methods, carbon monoxide reaction preparation, organic compound/hydride/coordination complex catalysts, etc. It can solve the problems of low positive-to-negative ratio and poor catalyst stability, etc. , to achieve the effect of prolonging service life, high catalytic activity and reducing energy consumption of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
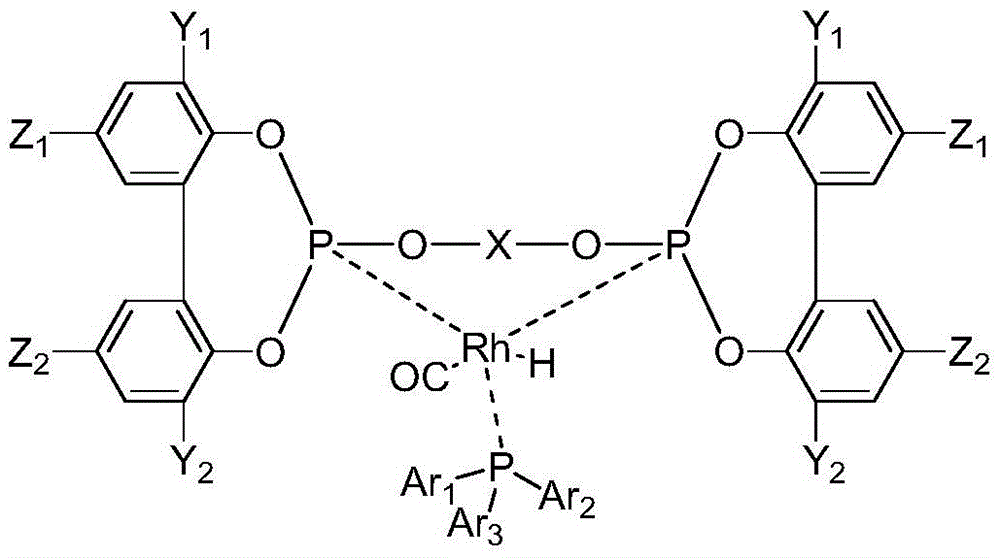
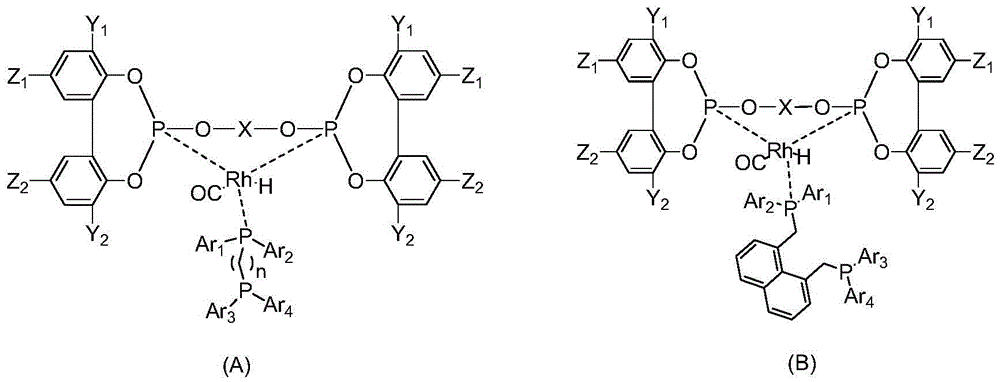
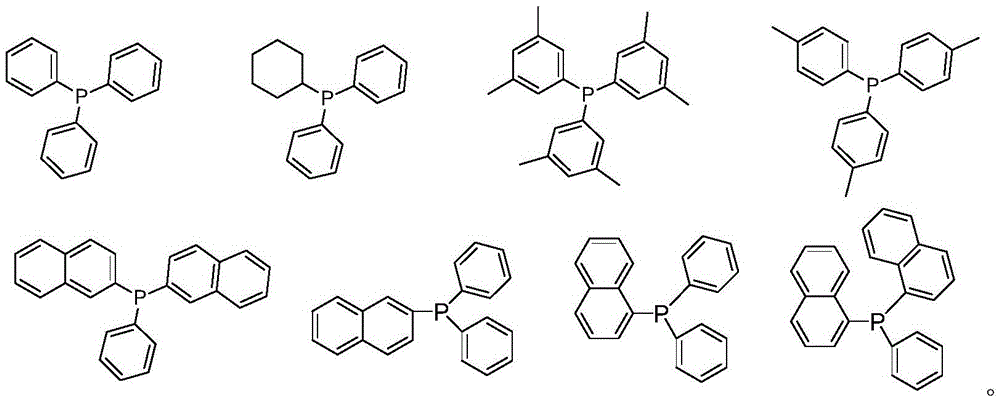
[0049] Add [Rh(acac)(CO) to a 200mL stainless steel autoclave equipped with a pressure gauge under air atmosphere 2 ] (0.07mmol, 18.1mg) and 0.28mmol of triphenylphosphine L1 monophosphine ligand and 0.14mmol of L12 bidentate phosphite ligand, and 70mL of anhydrous toluene, connect the gas line to synthesis gas (hydrogen: Carbon monoxide = 1:1) After replacing the gas in the kettle three times, stir with an electromagnetically driven mechanical stirrer, heat up to the inner temperature of the kettle at 100°C, and feed synthesis gas until the total pressure is 2.0MPa, at 100°C, 2.0MPa After reacting for 1 h, the rhodium / (bidentate phosphite-monophosphine ligand) complex catalyst HRh(L12)(L1)(CO) was obtained. Its molecular structure is as follows:
[0050] 31 PNMR (Toluene, 162MHz) P1 coordinated by L12 and rhodium: δ177.9ppm, P2 coordinated by L12 and rhodium: δ175.1ppm, P3 coordinated by L1 and rhodium: 36.2ppm, 1 J RhP1 = 249Hz, 1 J RhP2 = 249Hz, 1 J RhP3 = 141Hz, ...
Embodiment 2
[0052] Add [Rh(acac)(CO) to a 200mL stainless steel autoclave equipped with a pressure gauge under air atmosphere 2 ] (0.07mmol, 18.1mg) and 0.28mmol of triphenylphosphine L1 monophosphine ligand and 0.14mmol of L11 bidentate phosphite ligand, and 70mL of anhydrous toluene, connect the gas line to synthesis gas (hydrogen: Carbon monoxide = 1:1) After replacing the gas in the kettle three times, stir with an electromagnetically driven mechanical stirrer, heat up to the inner temperature of the kettle at 100°C, and feed synthesis gas until the total pressure is 2.0MPa, at 100°C, 2.0MPa After reacting for 1 h, the rhodium / (bidentate phosphite-monophosphine ligand) complex catalyst HRh(L11)(L1)(CO) was obtained. Its molecular structure is as follows:
[0053] P1 coordinated by L11 and rhodium: δ177.0ppm, P2 coordinated by L11 and rhodium: δ173.8ppm, P3 coordinated by L1 and rhodium: 34.3ppm, 1 J RhP1 = 251Hz; 1 J RhP2 = 246Hz, 1 J RhP3 = 141Hz, 2 J P1P2 = 279Hz, 2 J P...
Embodiment 3
[0055] Add [Rh(acac)(CO) to a 200mL stainless steel autoclave equipped with a pressure gauge under air atmosphere 2 ] (0.07mmol, 18.1mg) and the L4 monophosphine ligand of 0.28mmol and the L12 bidentate phosphite ligand of 0.14mmol, and 70mL anhydrous toluene, connect the gas line, with synthesis gas (hydrogen: carbon monoxide=1: 1) After replacing the gas in the kettle three times, stir with an electromagnetically driven mechanical stirrer, heat up to 100°C inside the kettle, feed synthesis gas until the total pressure is 2.0MPa, and react at 100°C and 2.0MPa for 1 hour, The rhodium / (bidentate phosphite-monophosphine ligand) complex catalyst HRh(L12)(L4)(CO) was obtained. Its molecular structure is as follows:
[0056] 31 PNMR (Toluene, 162MHz) P1 coordinated by L12 and rhodium: δ177.5ppm, P2 coordinated by L12 and rhodium: δ175.4ppm, P3 coordinated by L4 and rhodium: 34.4ppm, 1 J RhP1 = 248Hz, 1 J RhP2 = 249Hz, 1 J RhP3 = 139Hz, 2 J P1P2 = 282Hz, 2 J P1P3 = 157...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com