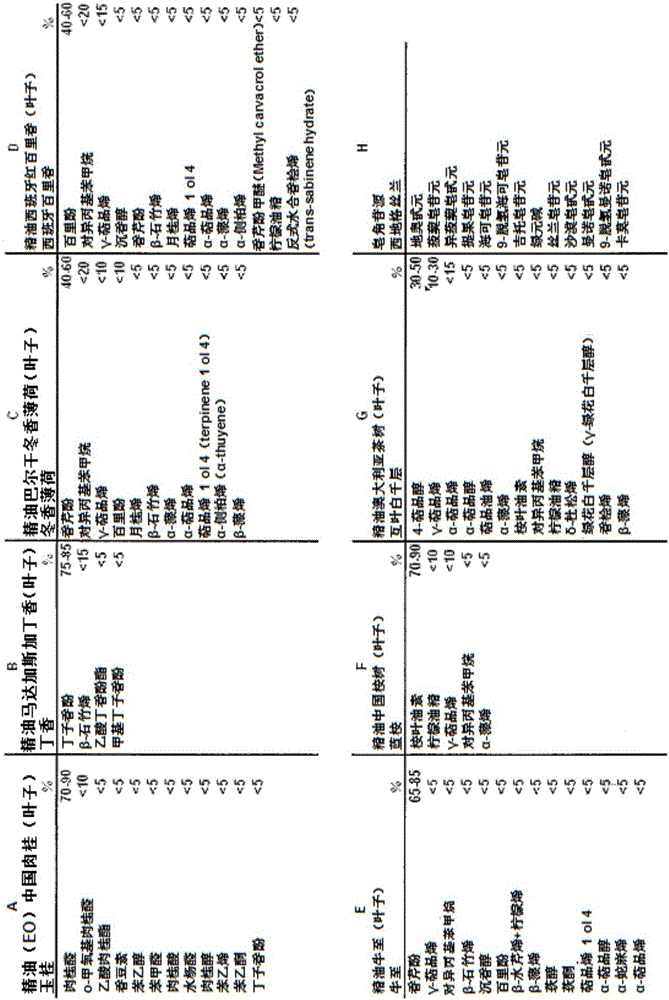
Oral anti-parasitic composition
A technology of antiparasitic drugs and compositions, which is applied in the direction of drug combinations, effective ingredients of hydroxyl compounds, anti-infective drugs, etc., and can solve problems such as skin damage and skin irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
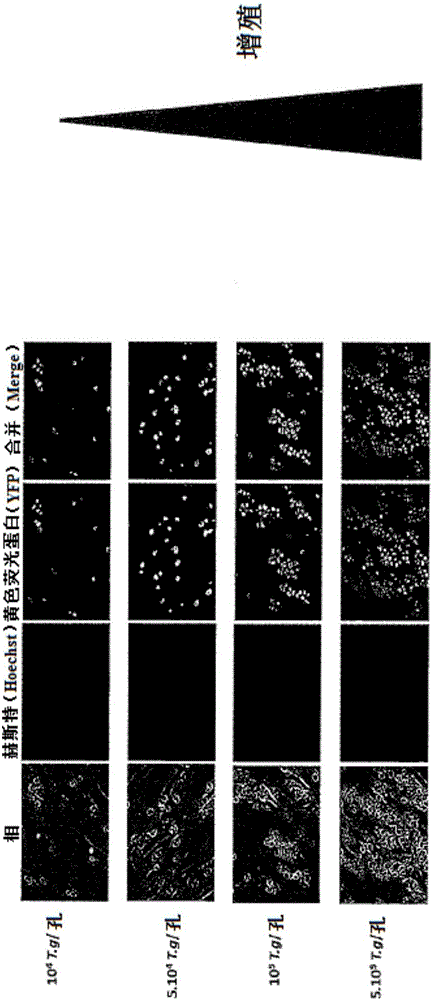
[0080] Example 1: Proliferation assay of the N. caninum parasite in the presence of essential oils
[0081] Proliferation of the parasite in the cells was determined by calculating the specific radioactivity of added tritiated uracil and compared to a control without essential oil (100% proliferation). The assay is performed in 24-well plates and the entire assay lasts approximately 24 hours. A positive control was obtained using pyrimethamine (IC50: 0.1 μg / mL). image 3 The IC50 for each essential oil solution tested at different concentrations in the presence of N. caninum is shown.
[0082] Table 1 (below) outlines image 3 The result of embodiment 1 in. Values indicate inhibition of proliferation. The dotted results show the IC50 for each essential oil tested.
[0083]
Embodiment 2
[0085] Toxoplasma gondii and Neospora caninum Inhibition of proliferation was determined by the addition of tritiated uracil, using the MTT assay Assess the viability of HFF cells. The results are shown in Figure 4 middle.
[0086] Figure 4 It was shown that various essential oils maintain cell viability HFF close to 80% at concentrations that have proliferation inhibitory properties against various parasites.
[0087] Insecticidally active oils could be observed with IC50 concentrations obtained for T. gondii and N. caninum. However, F oil and G oil had little or no effect on proliferation inhibition of N. caninum.
Embodiment 3
[0088] Example 3: Proliferation assay of Toxoplasma gondii in the presence of a combination of essential oils
[0089] The groups of essential oil combinations are: AB, AE, AD and BE.
[0090] A 3×3 factorial design was adopted for each combination. Theoretical IC50s were adjusted to the measured rates of inhibition of proliferation, which were then used as a basis for making dilutions and subsequently combining 9 different ratios of the 2 essential oils in the mixture.
[0091]
[0092] For each combination, 9 T. gondii proliferation inhibition assays were performed (all data not shown). Again, inhibition of proliferation was determined by addition of tritiated uracil.
[0093] Mixture BE (see Figure 5 ): shows that the mixture BE has a synergistic effect on the proliferation of Toxoplasma gondii.
[0094] Only 100 ppm of essential oil B corresponds to IC58 (58% inhibition of proliferation).
[0095] Only 75 ppm of essential oil E corresponds to IC88 (88% inhibitio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com