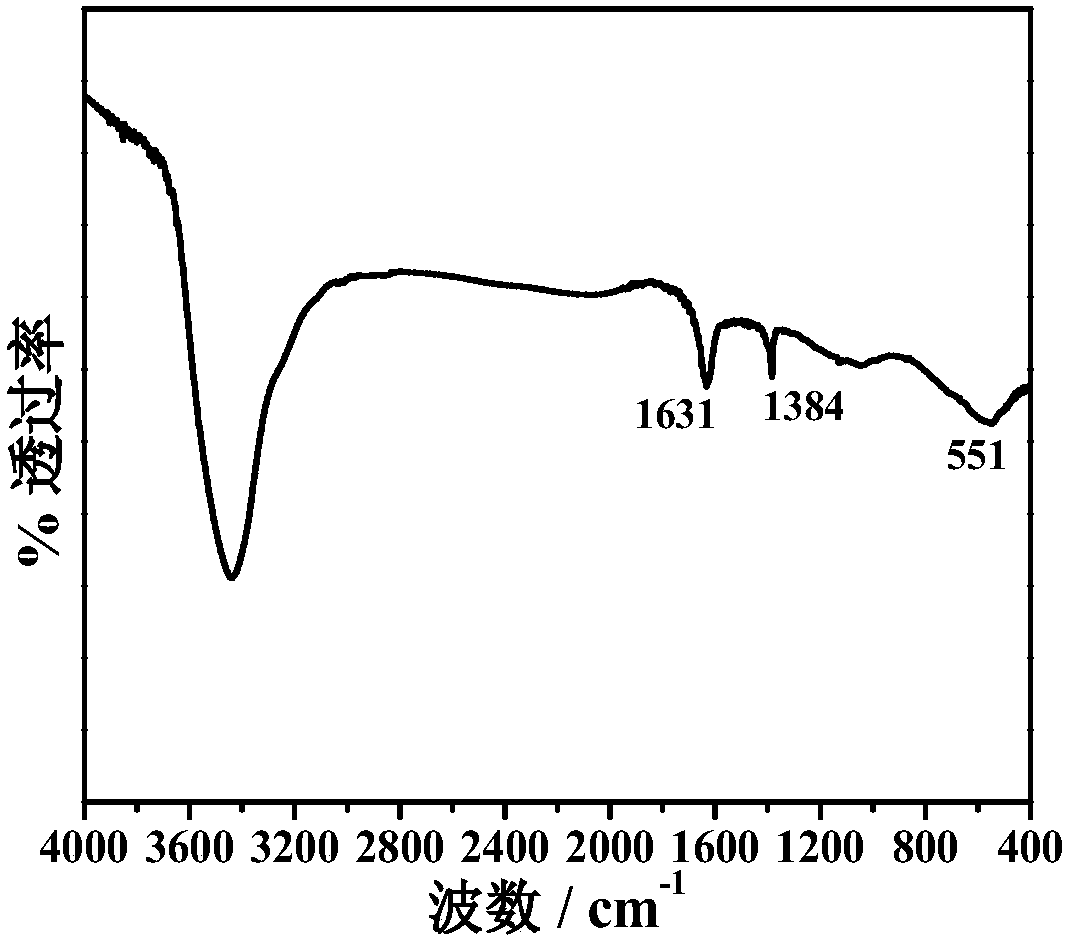
One-step synthesis of sulfur-doped graphene airgel and its electrosorption removal of various heavy metal ions
A technology of sulfur-doped graphene and heavy metal ions, which is applied in the direction of graphene, separation methods, and dispersed particle separation, to achieve high adsorption efficiency, improved electro-adsorption performance, and environmentally friendly and pollution-free preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of sulfur-doped graphene airgel material paper electrode includes the following steps:
[0022] (1) Ultrasonic disperse 0.15g of graphene oxide (GO) in 100mL of distilled water, then add 0.45g of dithiothreitol, stir it mechanically to make it fully mixed, and then place the mixture in a water bath at 95°C Heat for 2h. After the reaction, the resulting product was immersed in distilled water for 2 to 3 days, and finally the sample was freeze-dried at -52°C for 24 hours to obtain the sulfur-doped graphene airgel.
[0023] (2) Add 90mg of the sulfur-doped graphene airgel material prepared in step (1) into 2mL of 4wt% polyvinyl alcohol solution, and ultrasonically disperse the composite material in the solution evenly. Take 0.16mL of the above dispersion liquid and apply it evenly on a 35mm×8mm hard paper sheet (thickness 400μm), and freeze-dry it at -52°C for 12h to make a sulfur-doped graphene airgel paper electrode.
Embodiment 2
[0025] The preparation process of the sulfur-doped graphene airgel paper electrode is the same as that of Example 1.
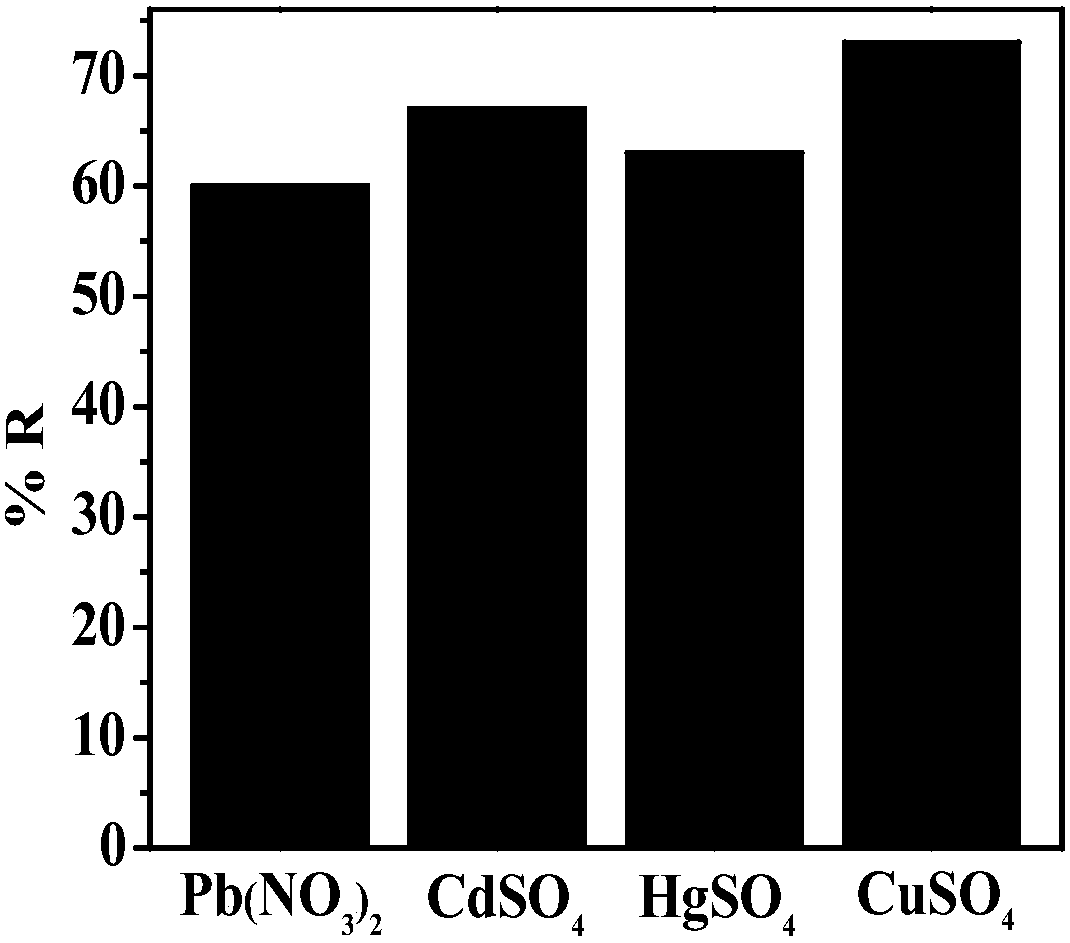
[0026] The prepared sulfur-doped graphene airgel paper electrodes were used for Pb(NO 3 ) 2 , CdSO 4 , HgSO 4 and CuSO 4 Electrochemical treatment of the solution, the applied voltage is -0.3V, the treatment time is 2min, Pb 2+ 、Cd 2+ , Hg 2+ and Cu 2+ The removal rate see figure 2 , it can be seen that the sulfur-doped graphene airgel material has a strong effect on the heavy metal ion Cu 2+ The adsorption effect is the best.
Embodiment 3
[0028] The preparation process of the sulfur-doped graphene airgel paper electrode is the same as that of Example 1.
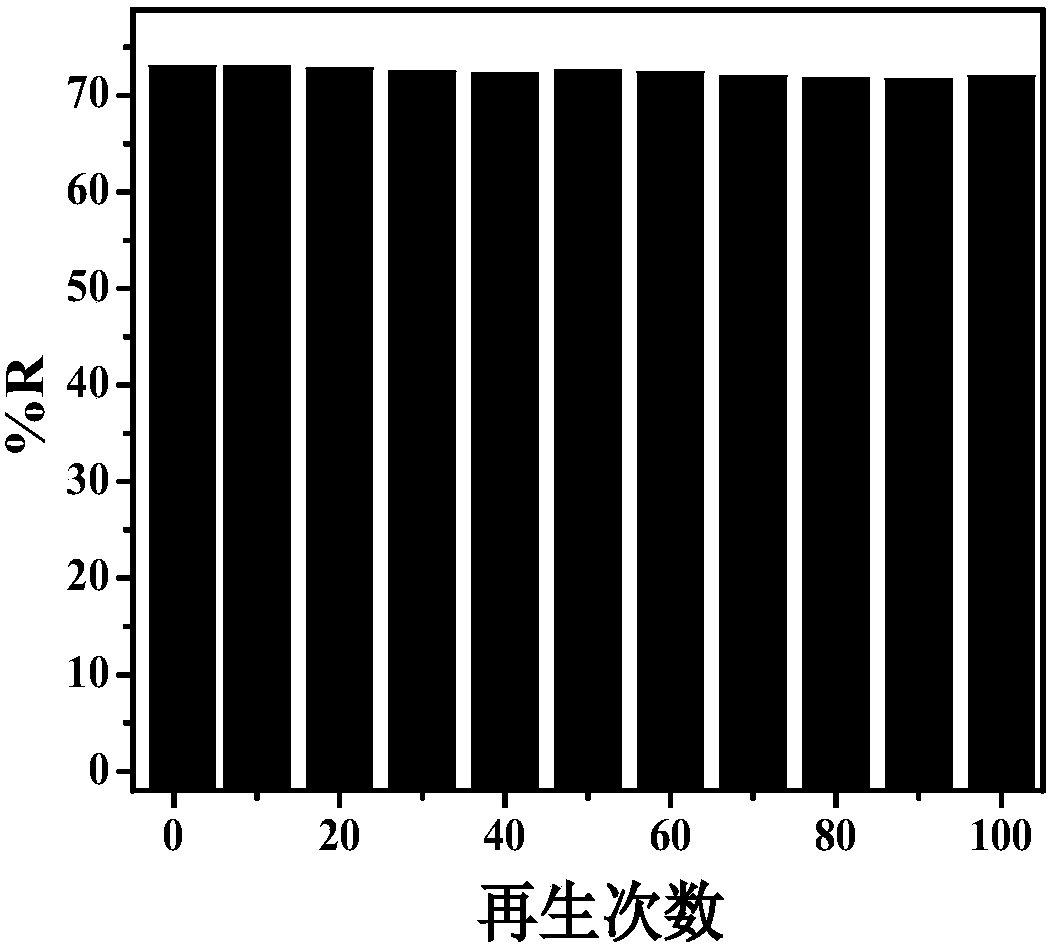
[0029] Cyclic electrosorption experiments on sulfur-doped graphene airgel paper electrodes. to Cu 2+ Taking the electrosorption as an example, a cyclic electrosorption experiment was carried out on the sulfur-doped graphene airgel paper electrode. Place the sulfur-doped graphene airgel paper electrode in 80 mL of CuSO with a concentration of 1 mmol / L 4 In the solution, apply a potential of -0.3V, and record the conductivity of the solution, record the conductivity of the solution again after 2 minutes, and calculate the removal rate. Then the potential was removed to allow it to desorb, and the cycle was repeated several times. Experimental results such as image 3 shown. Adsorbed Cu for the first time 2+ The removal rate was 73%, and after 100 cycles of use, the electrode paired Cu 2+ The removal rate was 70.5%. It shows that the material has extremel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com