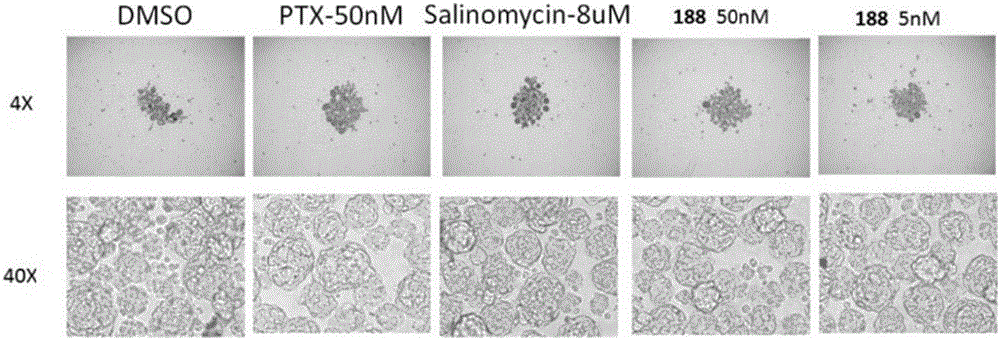
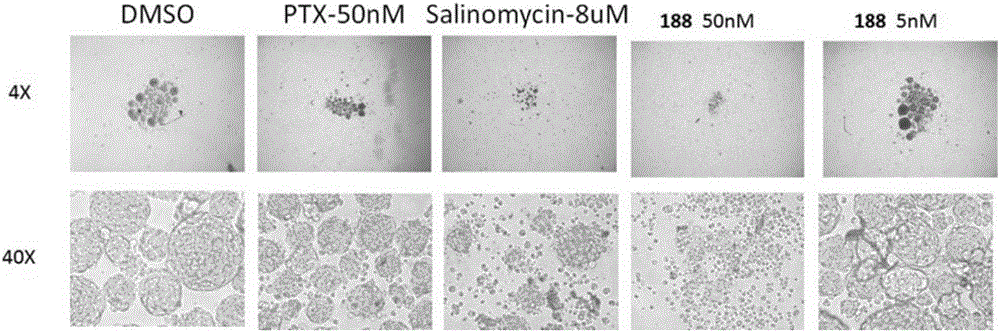
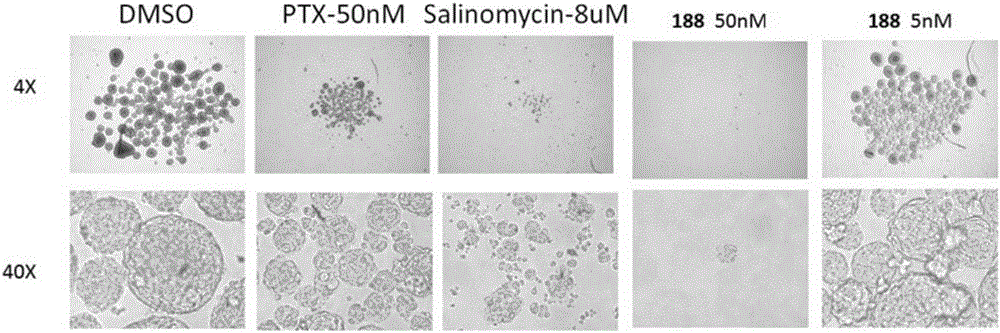
Compound and application thereof
A compound and solvate technology, which is applied in the treatment of proliferative diseases, especially in the field of cancer, can solve the problems of high inhibitory activity and achieve the effect of inhibiting regeneration and strongly inhibiting the proliferation of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Example 1 (S)-2-[7-(3,5-Dimethylphenoxy)-2,5-dioxo-8-(3,4,5-trimethoxybenzamido)-2, Methyl 3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-3-yl]acetate (compound 1)
[0193] Preparation of 5-chloro-2,4-dinitrobenzoic acid (Intermediate 188): Dissolve 3-chlorobenzoic acid (10.0 g, 63.9 mmol) in 120 mL of concentrated sulfuric acid with stirring at room temperature, and add nitric acid in batches within 15 min Potassium (16.5 g, 163.2 mmol). The reaction solution was reacted at 80°C for 30min, 110°C for 2h, and 120°C for 2h. The reaction solution was poured into 660 g of ice water, filtered, and the resulting white solid was recrystallized with a mixed solvent of ethanol and water to obtain 8.08 g of light yellow crystals, with a yield of 51.3%. 1 HNMR (400MHz, DMSO-d 6 )δ14.21(brs,1H),8.85(s,1H),8.28(s,1H). 13 CNMR (100MHz, DMSO-d 6 )δ163.78, 147.95, 145.70, 132.86, 132.06, 130.52, 122.05.
[0194] Preparation of 5-(3,5-dimethylphenoxy)-2,4-dinitrobenzoic acid (Interme...
Embodiment 2
[0198] Example 2 (S)-2-[8-(3,5-dimethoxybenzamido)-7-(3,5-dimethylphenoxy)-2,5-dioxo-2,3 ,4,5-Tetrahydro-1H-benzo[e][1,4]diazepine-3-yl]acetic acid methyl ester (compound 2)
[0199] According to the method described in Example 1, the title compound (2) was prepared using intermediate 191 (191.7 mg, 0.5 mmol) and 3,5-dimethoxybenzoyl chloride (200 mg, 1.0 mmol). Mp137–139°C. 1 HNMR (300MHz, DMSO-d 6 )δ10.54(s,1H),9.87(s,1H),8.60(d,J=5.1Hz,1H),7.71(s,1H),7.22(s,1H),6.94(d,J=2.2 Hz,2H),6.80(s,1H),6.73–6.66(m,3H),4.14(dt,J=8.7,5.6Hz,1H),3.78(s,6H),3.60(s,3H),2.90 (dd,J=17.0,8.7Hz,1H),2.74(dd,J=17.0,5.8Hz,1H),2.24(s,6H). 13 CNMR (100MHz, DMSO-d 6 )δ170.61,170.56,166.85,165.29,160.48,156.19,145.59,139.54,136.18,132.97,132.43,125.58,123.19,119.62,117.31,116.23,105.71,103.79,55.52,51.65,48.68,32.53,20.91.HRMScalcd.forC 29 h 30 N 3 o 8 (M+H + )548.2033; found548.2027
Embodiment 3
[0200] Example 3(S)-2-[7-(3,5-Dimethylphenoxy)-2,5-dioxo-8-(2,3,4-trimethoxybenzamido)-2, 3,4,5-Tetrahydro-1H-benzo[e][1,4]diazepin-3-yl]acetic acid methyl ester (compound 3)
[0201] According to the method described in Example 1, the title compound (3) was prepared using intermediate 191 (191.7 mg, 0.5 mmol) and 2,3,4-trimethoxybenzoyl chloride (230.6 mg, 1.0 mmol). Mp136–138°C. 1 HNMR (300MHz, DMSO-d 6 )δ10.96(s,1H),10.53(s,1H),8.55(d,J=5.1Hz,1H),8.49(s,1H),7.86(d,J=9.0Hz,1H),7.10( s,1H),7.05(d,J=9.2Hz,1H),6.90(s,1H),6.85(s,2H),4.10(dt,J=8.7,5.5Hz,1H),3.89(s,3H ),3.87(s,3H),3.77(s,3H),3.59(s,3H),2.89(dd,J=17.0,8.8Hz,1H),2.72(dd,J=17.0,5.8Hz,1H) ,2.30(s,6H). 13 CNMR (100MHz, DMSO-d 6 )δ170.63,170.47,166.83,162.29,157.24,155.65,151.89,142.93,141.34,139.98,133.24,132.67,126.45,126.34,120.91,117.52,117.39,116.83,112.34,108.54,61.97,60.61,56.19,51.60,48.62 ,32.50,20.93.HRMScalcd.forC 30 h 32 N 3 o 9 (M+H + )578.2139; found578.2116.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com