A bivalent anthrax vaccine
An anthrax and vaccine technology, applied in the field of immunomedicine, can solve problems such as lethal toxicity and achieve good immune effect, enhanced protective effect, excellent immunogenicity and protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
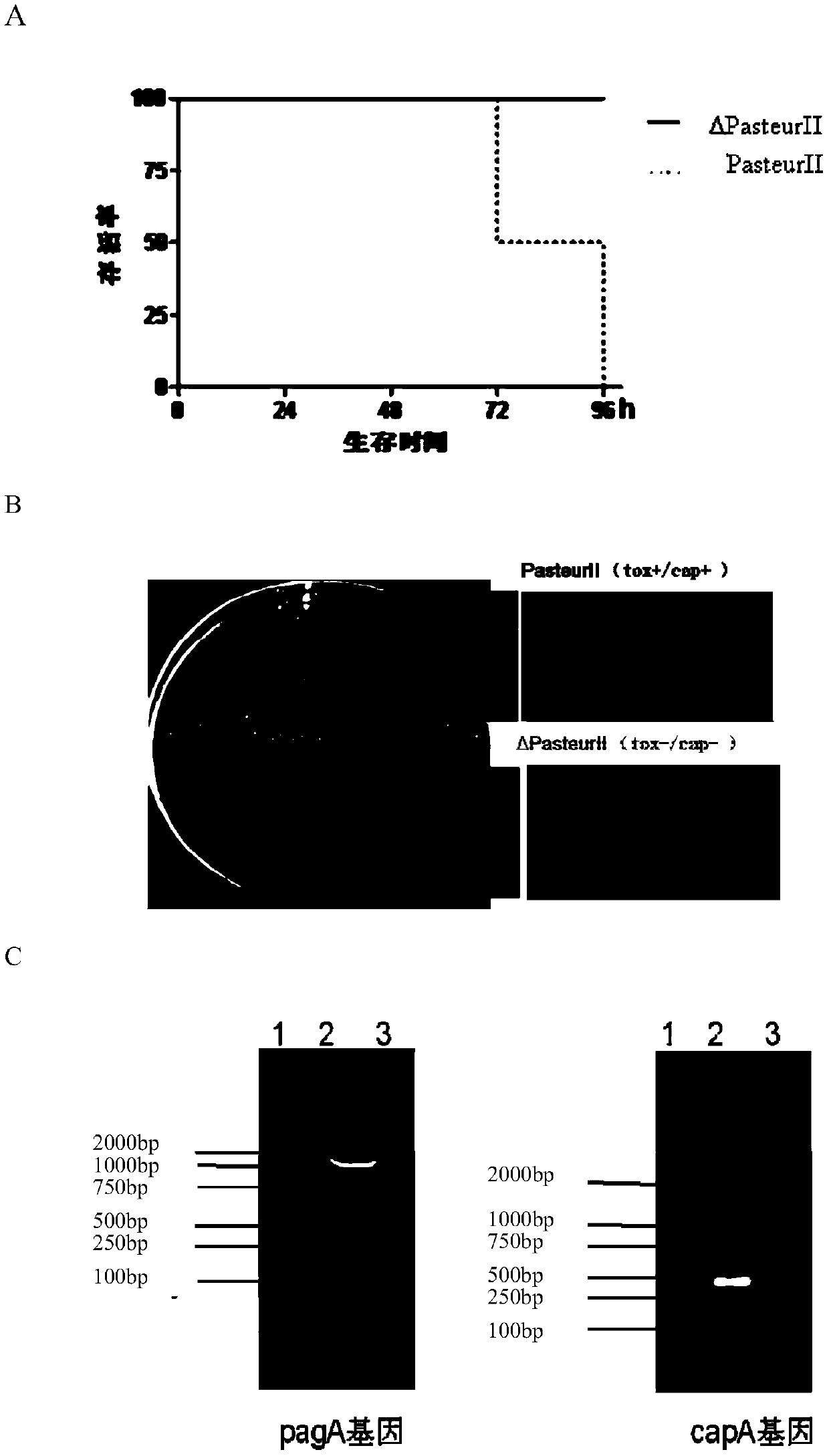
[0055] 1. Obtain a plasmid-free ΔPasteurII recipient strain (tox - ,cap - )
[0056] Bacillus anthracis Pasteur II strain (tox + / cap + ) The vaccine strain was subcultured at 37°C with shaking in a medium containing novobiocin (1 μg / μl), and the pXO2 plasmid was deleted. Then in 0.05% SDS medium containing 42 ℃ shaking continuous subculture, delete the pXO1 plasmid, obtain a strain of Bacillus anthracis completely eliminate the plasmid pXO1 and pXO2, named ΔPasteurII strain (tox - / cap - ). The results of animal toxicity tests were as follows: figure 1 As shown in A, it is confirmed that the ΔPasteurII strain has lost its virulence; capsule staining is as follows figure 1 As shown in B, it shows that the ΔPasteurII strain loses the capsule; the PCR reaction of the PagA gene on pXO1 and the capA gene on pXO2 is as follows figure 1 Shown in C, demonstrating that the ΔPasteurII strain was deleted (pXO1 - , pXO2 - ) plasmid; Western-blotting protective antigen-antibody ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com