Preparation method and application of mannose grafted trimethyl chitosan
A technology of trimethyl chitosan and mannose, which is applied in the direction of non-active ingredient medical preparations, pharmaceutical formulas, block delivery, etc., can solve the problems of low absorption rate and poor immunogenicity, and achieve simple processing and improved Drug activity, effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
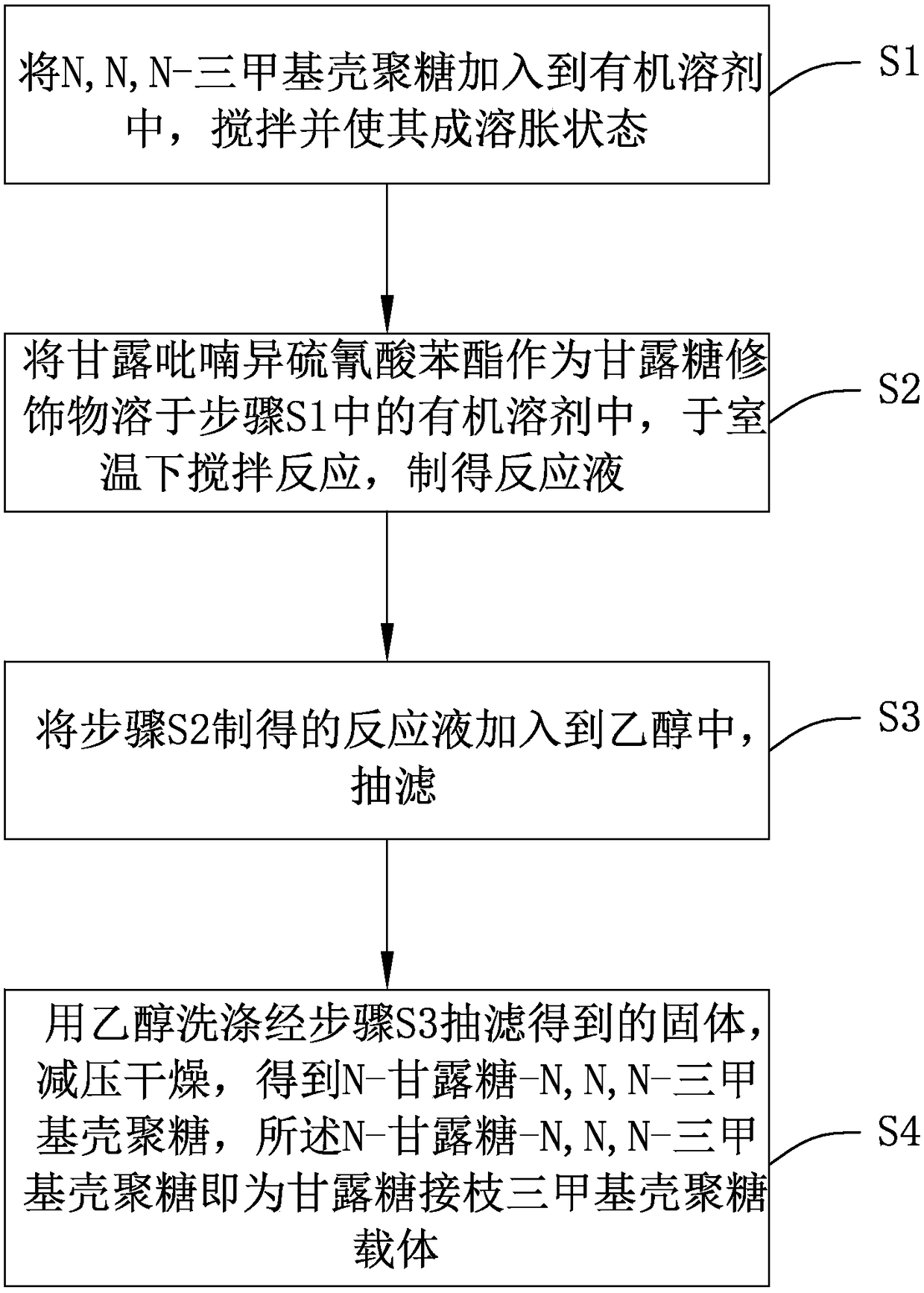
[0041] see figure 1 , a flow chart of the preparation method of the mannose-grafted trimethyl chitosan carrier provided by the invention. The preparation method of described mannose grafting trimethyl chitosan carrier, comprises the following steps:
[0042] Step S1, adding N,N,N-trimethyl chitosan into the organic solvent, stirring and making it into a swollen state;
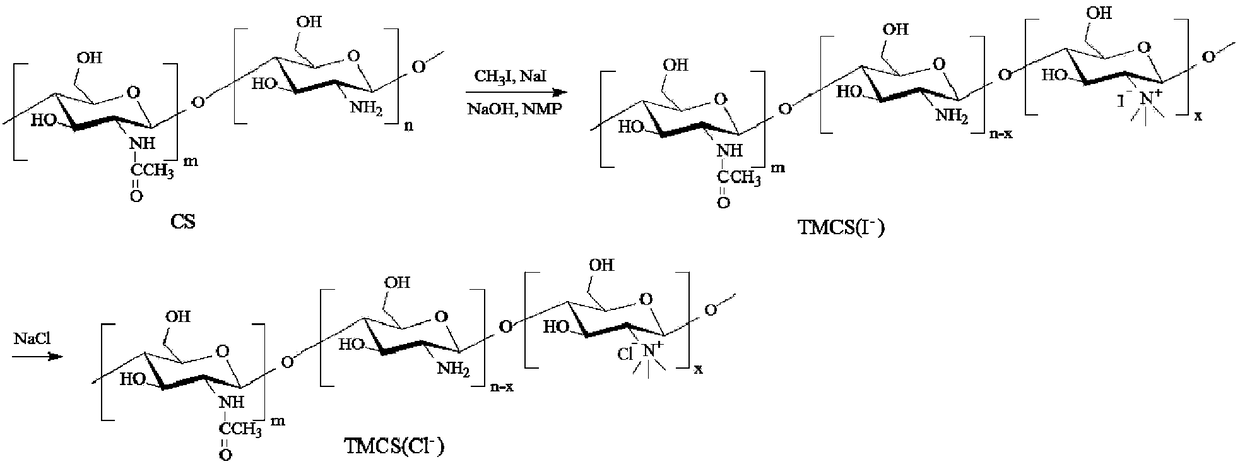
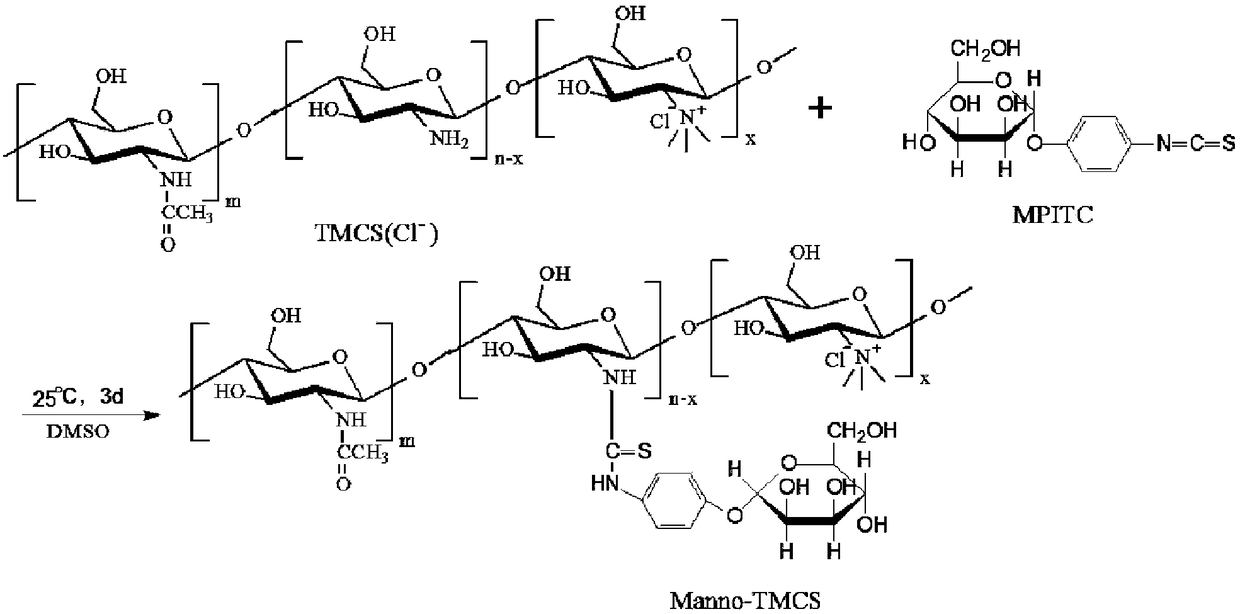
[0043] Specifically, see figure 2 , is the synthetic route map of N,N,N-trimethyl chitosan. Put 2g of chitosan (chitosan, CS) with a molecular weight of 20kDa-1000kDa and 80mL of N-methyl-2-pyrrolidone (N-methyl-2-pyrrolidone, NMP) into a round bottom flask, or use dimethylformamide and N -Any one or more combinations of organic solvents such as methylpyrrolidone, stirred overnight at room temperature to swell chitosan, added 4.8g of sodium iodide and 11mL of 15% NaOH solution, stirred at 60°C for 20 minutes, added 12mL of iodine methane, continue to react for 2 hours; add 11mL of 15% NaOH solution and 6mL o...
Embodiment 2
[0061] Weigh 10 mg Manno-TMCS and 5 mg ovalbumin (Ovalbumin, OVA), dissolve in 5 mL deionized water, and prepare a Manno-TMCS-OVA solution. Weigh 2 mg of sodium alginate (Sodium alginate, SL), dissolve it in 10 mL of deionized water, and prepare a 0.2 mg / mL SL solution. Under magnetic stirring, add 2 mL of SL solution dropwise to Manno-TMCS-OVA solution at a rate of 6 mL / h, continue stirring for 30 min after the addition is complete, refrigerate and centrifuge to collect the precipitate, wash, and dry to obtain mannose-grafted trimethyl Chitosan-loaded ovalbumin microspheres.
Embodiment 3
[0063] Weigh 10 mg Manno-TMCS and 5 mg ovalbumin (OVA), dissolve in 5 mL deionized water, and prepare Manno-TMCS-OVA solution. Weigh 4 mg of sodium alginate, dissolve it in 10 mL of deionized water, and prepare a 0.4 mg / mL SL solution. Under magnetic stirring, add 2 mL of SL solution dropwise to Manno-TMCS-OVA solution at a rate of 6 mL / h, continue stirring for 30 min after the addition is complete, refrigerate and centrifuge to collect the precipitate, wash, and dry to obtain mannose-grafted trimethyl Chitosan-loaded ovalbumin microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com