Preparation method of mannose-grafted trimethyl chitosan and application of preparation method
A technology of trimethyl chitosan and mannose, which is applied in the directions of non-active ingredients such as medical preparations, pharmaceutical formulas, bulk delivery, etc. Effects of drug activity, ability to enhance drug transmembrane transport and paracellular transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
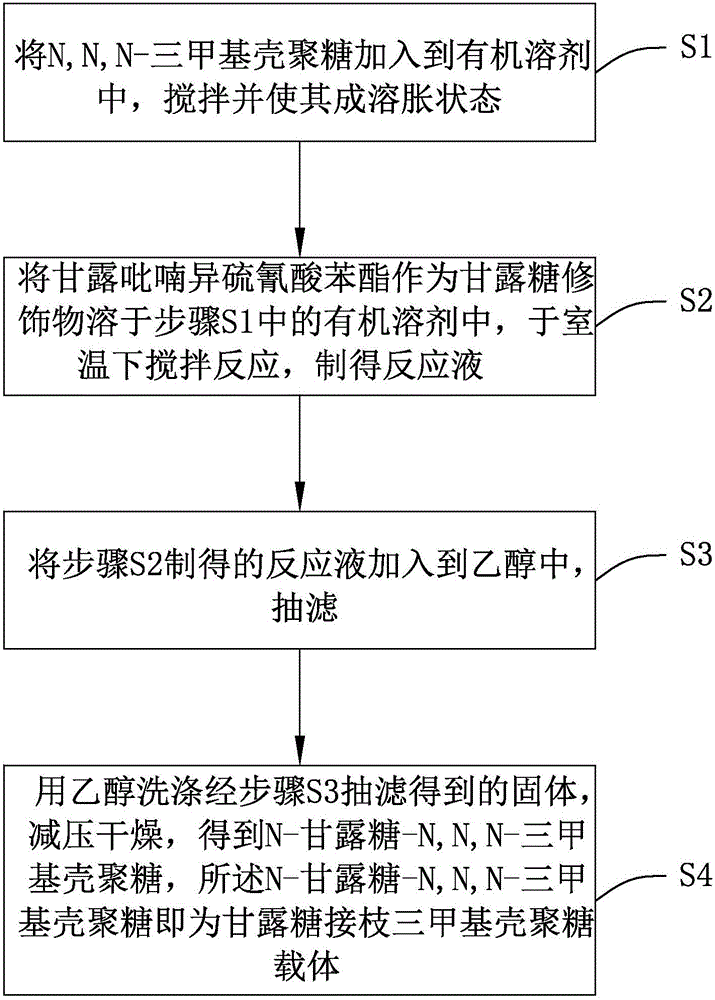
[0041] see figure 1 , a flow chart of the preparation method of the mannose-grafted trimethyl chitosan carrier provided by the invention. The preparation method of described mannose grafting trimethyl chitosan carrier, comprises the following steps:
[0042] Step S1, adding N,N,N-trimethyl chitosan into the organic solvent, stirring and making it into a swollen state;
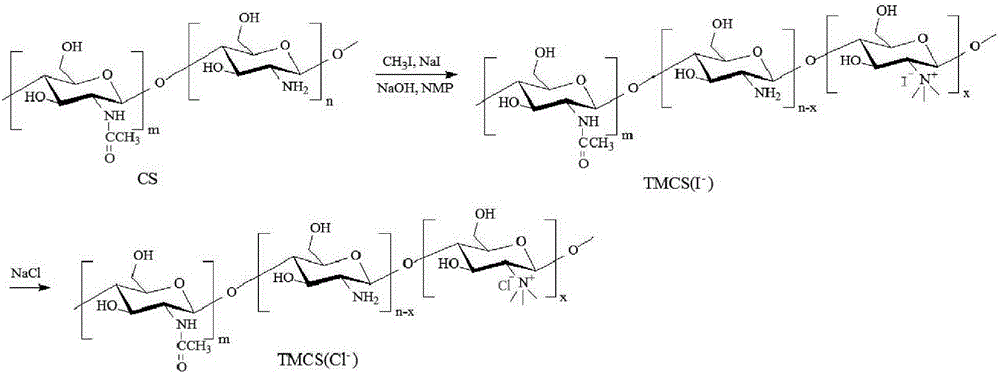
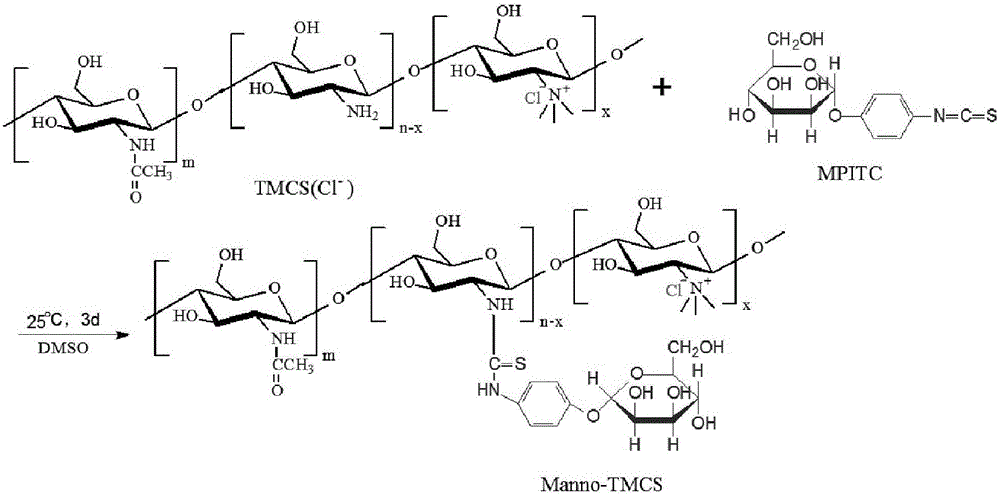
[0043] Specifically, see figure 2 , is the synthetic route map of N,N,N-trimethyl chitosan. Put 2g of chitosan (chitosan, CS) with a molecular weight of 20kDa-1000kDa and 80mL of N-methyl-2-pyrrolidone (N-methyl-2-pyrrolidone, NMP) into a round bottom flask, or use dimethylformamide and N- Any one or more combinations of organic solvents such as methylpyrrolidone, stirred overnight at room temperature to swell chitosan, added 4.8g sodium iodide and 11mL15% NaOH solution, stirred at 60°C for 20 minutes, added 12mL methyl iodide , continue to react for 2 hours; add 11mL of 15% NaOH solution and 6mL of methyl i...
Embodiment 2
[0061] Weigh 10 mg Manno-TMCS and 5 mg ovalbumin (Ovalbumin, OVA), dissolve in 5 mL deionized water, and prepare a Manno-TMCS-OVA solution. Weigh 2 mg of sodium alginate (Sodiumalginate, SL), dissolve it in 10 mL of deionized water, and prepare a 0.2 mg / mL SL solution. Under magnetic stirring, add 2mL of SL solution to Manno-TMCS-OVA solution dropwise at a rate of 6mL / h. After the dropwise addition, continue to stir for 30min, collect the precipitate by refrigerated centrifugation, wash, and dry to obtain mannose-grafted trimethyl Chitosan loaded ovalbumin microsphere preparation.
Embodiment 3
[0063] Weigh 10 mg Manno-TMCS and 5 mg ovalbumin (OVA), dissolve in 5 mL deionized water, and prepare Manno-TMCS-OVA solution. Weigh 4 mg of sodium alginate, dissolve it in 10 mL of deionized water, and prepare a 0.4 mg / mL SL solution. Under magnetic stirring, add 2mL of SL solution to Manno-TMCS-OVA solution dropwise at a rate of 6mL / h. After the dropwise addition, continue to stir for 30min, collect the precipitate by refrigerated centrifugation, wash, and dry to obtain mannose-grafted trimethyl Chitosan loaded ovalbumin microsphere preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com