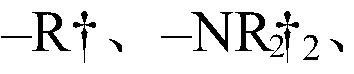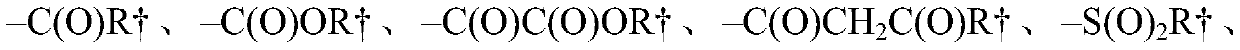Substituted gemcitabine bicyclic amide analogs and treatment methods using same
A technology of aromatic rings and compounds, applied in the field of substituted gemcitabine arylamide analogues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0379] The disclosed preparation methods may provide compounds which may contain one or more asymmetric centers and thus may give rise to enantiomers and diastereomers. Unless stated to the contrary, the compounds prepared by the disclosed methods include all such possible diastereoisomers and their racemic mixtures, their substantially pure resolved enantiomers, all possible geometric isomers Constructs and pharmaceutically acceptable salts thereof. Also included are mixtures of stereoisomers as well as isolated specific stereoisomers.
[0380] In one aspect, the disclosed preparation methods can provide racemic mixtures resolved into pure or substantially pure enantiomers using chiral phase chromatography or other suitable methods known to those skilled in the art and non-racemic mixture. As is known to those skilled in the art, a variety of specific columns and / or mobile phases can affect the desired resolution of enantiomers, and the specific choice can be determined by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com