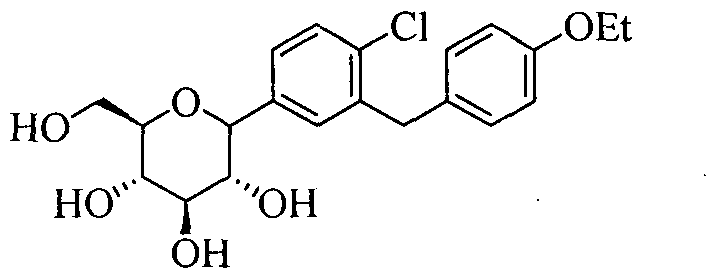
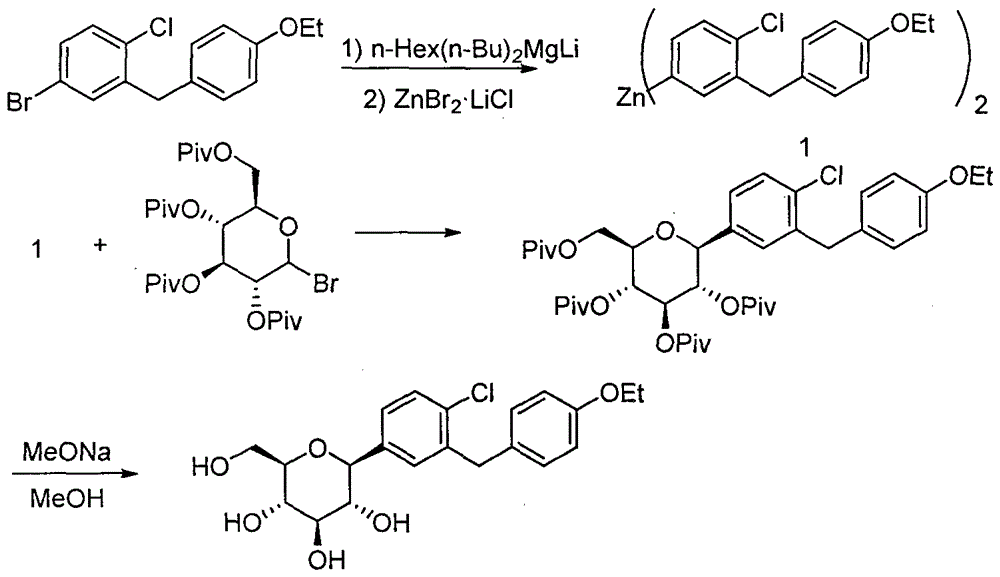
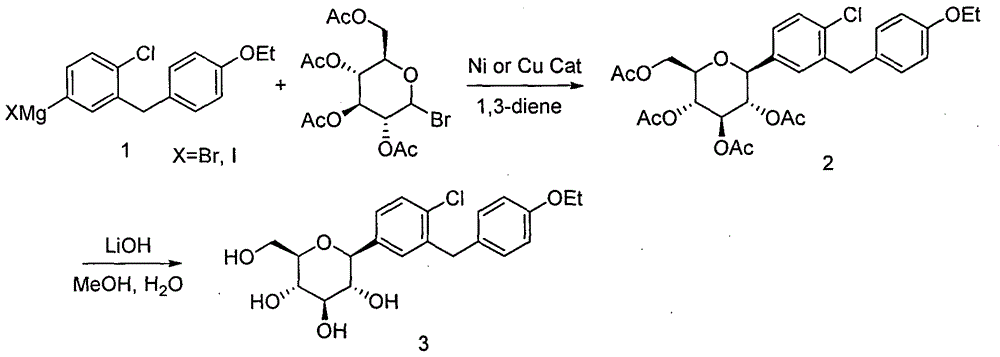
Synthesis method of dapagliflozin
A compound and Grignard reagent technology, applied in the field of chemical drug preparation, can solve the problems of large limitations, complex reaction products, low yield, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: 2,3,4,6-tetra-O-acetyl-1-[4-chloro-3-(4-ethoxybenzyl)phenyl]-1-deoxy-β-D-pyridine Synthesis of Glucopyranose (Compound 2)
[0026] Method 1): Take a 500ml three-neck reaction flask, stir it with magnetic force, add 56g (150mmol) of 4-iodo-1-chloro-2-(4-ethoxybenzyl)benzene and 250mL of anhydrous tetrahydrofuran, nitrogen protection, temperature Lowered to -10°C, slowly added isopropylmagnesium chloride (80 mL, 2M tetrahydrofuran solution), and reacted at -5°C for 2 h. Take another 1000mL three-necked flask, add 49g (120mmol) of 2,3,4,6-tetraacetoxy-a-D-bromoglucopyranose, 1.93g (15mmol) of nickel chloride, 5.1g of isoprene ( 75mmol) and 250mL of anhydrous tetrahydrofuran, under nitrogen protection, the temperature dropped to 0°C, slowly added the Grignard reagent (compound 1) prepared in the previous 500mL bottle, added within 1h, kept at 5°C to 10°C for 12h, and saturated Aqueous ammonium chloride solution was used to quench the reaction, the organic phase...
Embodiment 2
[0028] Example 2: Synthesis of 1-[4-chloro-3-(4-ethoxybenzyl)phenyl]-1-deoxy-β-D-glucopyranose (compound 3, dapagliflozin)
[0029] Take a 1000mL three-neck flask, add 58g (100mmol) of compound 2 crystals, 300mL of methanol and 200mL of tetrahydrofuran, stir mechanically, keep the temperature of the reaction solution at 0°C to 5°C, add dropwise 100mL of lithium hydroxide aqueous solution (containing 2.4g of lithium hydroxide, 200mmol). After dripping, it was raised to room temperature and reacted overnight. After the reaction was completed, the solvent was concentrated under reduced pressure to remove most of the solvent, extracted with ethyl acetate, the combined organic phases were washed with saturated brine, and concentrated under reduced pressure to obtain 38.8 g of a white solid (95% yield). 1 H-NMR (CD3OD, 400MHz): δ7.34~7.25(m, 3H), 7.08(d, J=8.8Hz, 2H), 6.78(d, J=8.8Hz, 2H), 4.10(d, J= 9.2Hz, 1H), 4.06~3.95(m, 4H), 3.88~3.85(m, 1H), 3.72~3.68(m, 1H), 3.47~3.37(m, 3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com