Three kinds of bio-based epoxy resins based on furan ring structure and their preparation methods and applications
A technology based on epoxy resin and furan ring, which is applied in the field of bio-based polymer materials, can solve the problems of poor heat resistance of materials, influence on material preparation, and decrease in mechanical properties of materials, and achieve the effects of reducing pollution, easy implementation and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Dissolve 10g of maleic anhydride and 4.8g of methanolamine in 10ml of tetrahydrofuran, react at 10°C for 72h, cool to room temperature overnight to precipitate a white precipitate, filter it with suction, wash with a small amount of ethanol, and dry it to obtain N-hydroxy Methylmaleimide. Then furan and N-methylolmaleimide were stirred at room temperature for 24h to obtain furan N-hydroxymethylmaleimide.
[0050](2) Dissolve 10 g of furan N-methylol maleimide and 4.3 g of acryloyl chloride in 5 ml of butyl acetate, and stir with a magnet. Subsequently, 0.48 g of triethylamine was dissolved in 5 ml of butyl acetate, and added dropwise through a constant pressure separatory funnel for 1 hour, and then the temperature was raised to 10° C. for 72 hours. After the reaction is complete, add 10ml of sodium bicarbonate aqueous solution and stir for half an hour to remove excess acryloyl chloride, then pour the mixed solution into a separatory funnel and wash with water thr...
Embodiment 2
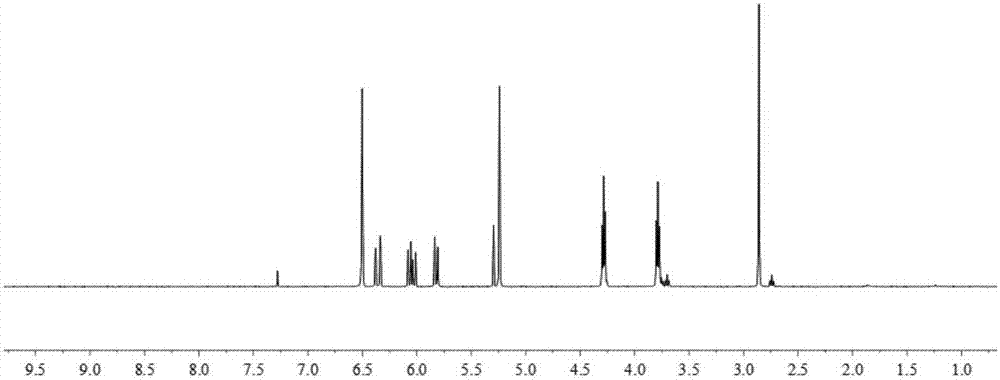
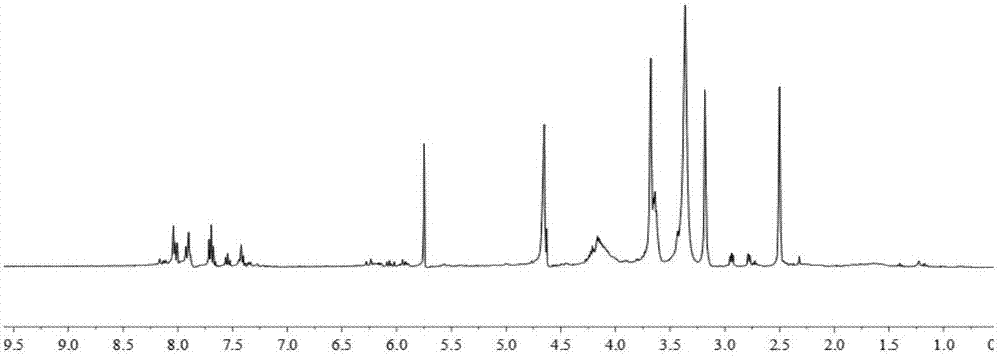
[0055] (1) Dissolve 10g of maleic anhydride and 14.4g of ethanolamine in 30ml of dioxane, react at 20°C for 65h, cool to room temperature overnight to precipitate a white precipitate, filter with suction, wash with a small amount of ethanol, and dry it to obtain N -Hydroxyethylmaleimide, 1 H-NMR such as image 3 As shown, each peak on the figure is in one-to-one correspondence with the hydrogen atoms on the N-hydroxyethylmaleimide structure, 13 C-NMR such as Figure 4 As shown, each peak on the figure corresponds to the carbon atom above the N-hydroxyethylmaleimide structure. Then furan and N-hydroxyethylmaleimide were stirred at room temperature for 24h to obtain furan N-hydroxyethylmaleimide.
[0056] (2) Dissolve 10 g of furan N-hydroxyethylmaleimide and 12.9 g of acryloyl chloride in 15 ml of ethanol, and stir with a magnet. Subsequently, 4.8 g of triethylamine was dissolved in 15 ml of ethanol, and added dropwise through a constant pressure separatory funnel for 1 hou...
Embodiment 3
[0061] (1) Dissolve 10g of maleic anhydride and 24g of ethanolamine in 50ml of ether, react at 30°C for 55h, cool to room temperature overnight to precipitate a white precipitate, filter it with suction, wash with a small amount of ethanol, and dry it to obtain N-hydroxypropyl maleimide. Then furan and N-hydroxypropylmaleimide were stirred at room temperature for 24h to obtain furan N-hydroxypropylmaleimide.
[0062] (2) Dissolve 10 g of furan N-hydroxypropylmaleimide and 21.5 g of acryloyl chloride in 25 ml of tetrahydrofuran, and stir with a magnet. Subsequently, 14.4 g of triethylamine was dissolved in 25 ml of tetrahydrofuran, added dropwise through a constant pressure separatory funnel, and the drop was completed in 1 hour, and then the temperature was raised to 30° C. for 55 hours. After the reaction is complete, add 30ml of sodium bicarbonate aqueous solution and stir for half an hour to remove excess acryloyl chloride, then pour the mixed solution into a separatory fu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| adhesivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com