Method for induced differentiation of human amniotic mesenchyme cells in vitro into insulin-secreting cells
An amniotic mesenchyme, insulin secretion technology, applied in the biological field, can solve the problems of not expressing telomerase gene, not having long-term self-renewal and the ability to generate single cell clones, and not being tumorigenic.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
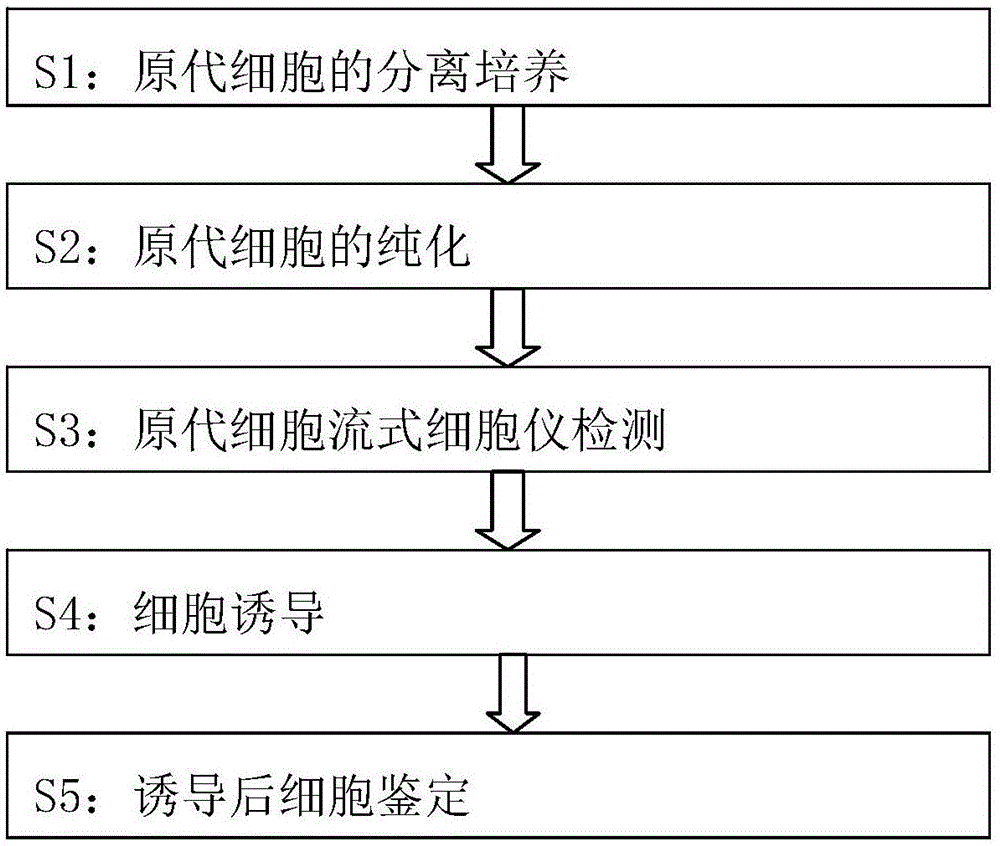
[0013] The method for directional differentiation of human amniotic mesenchymal cells into insulin-secreting cells in vitro comprises the following steps:
[0014] S1: Isolation and culture of primary cells: Under aseptic conditions, take fresh placenta discarded after delivery (with the consent of the family members), bluntly separate the amniotic membrane from the chorionic villi, and place it in a placenta containing double antibodies (100U / ML penicillin, 100UG / ML streptomycin). prime) D-HANKS solution for repeated washing. Cut the rinsed amniotic membrane into about 1*1mm pieces with ophthalmic scissors, add 2.5G / L trypsin to digest at 37°C for 10 minutes, add RPMI1640 medium containing 5% calf serum to stop the digestion, and gently blow and mix for 200 Filter with a mesh cell sieve, add 1.0g / L type Ⅱ collagenase digestion solution to the filtered amnion tissue, digest at 37°C for 0.5h, stop digestion again with 5% calf serum RPMI1640, gently blow and mix, and then use a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com