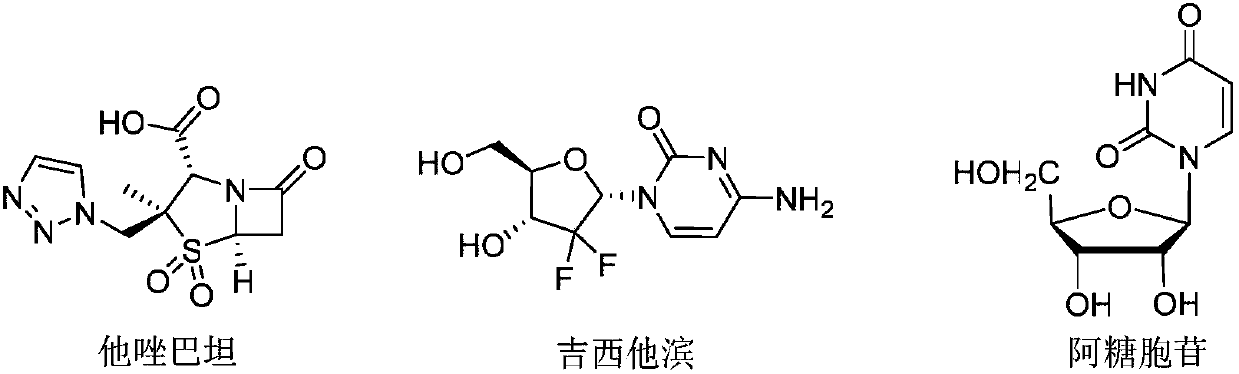
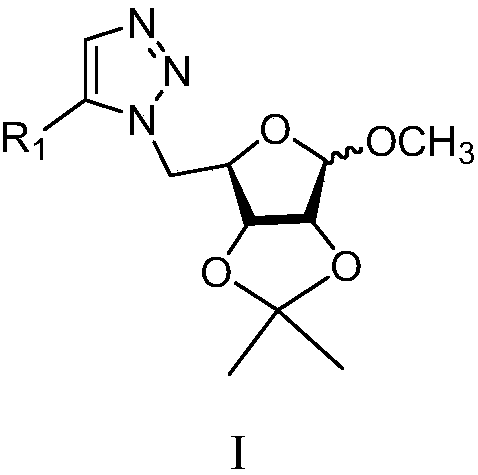
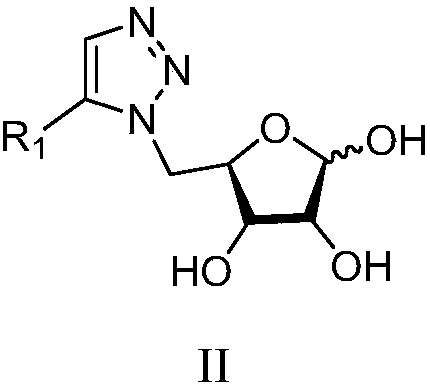
1,5-disubstituted 1,2,3-triazole sugar conjugates and their derivatives
A technology of triazole sugar and conjugate, which is applied in the field of 1,2,3-triazole sugar conjugate and achieves the effect of good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis.
[0026] Take the yellow needle-like solid nitrostyrene (100mg) into a 50mL round bottom flask, then add 10mL DMF as a solvent, and then add 1-methyl-2,3-O-isopropylidene-5-azido -D-ribofuranose (171mg), copper chloride (5mg) as a catalyst, react at 100°C, TLC dot plate to monitor the reaction process, after observing the completion of the reaction under ultraviolet light, lift the reaction flask from the oil bath, Cool to room temperature, extract with ethyl acetate / water system to obtain ethyl acetate layer, concentrate under reduced pressure to obtain crude product, and finally use ethyl acetate / petroleum ether solution with a volume ratio of 1:2 as eluent, silica gel column layer Analyzed and separated 203 mg of light yellow oily liquid with a yield of 91%. The resulting light yellow oil 1a was analyzed, and its proton nuclear magnetic resonance spectrum and carbon spectrum were as follows: 1 H NMR(600MHz, CDCl 3 )δ7.73(s,1H),7.52(m,J=8.4,2.0Hz,3...
Embodiment 2
[0027] Example 2: Synthesis
[0028] Take the yellow needle-like solid 2-chloro-nitrostyrene (100mg) into a 50mL round-bottomed flask, then add 10mL DMF as a solvent, and then add 1-methyl-2,3-O-isopropylene- 5-azido-D-ribofuranose (139mg), copper chloride (5mg) was used as a catalyst, and the reaction was carried out at 100°C. The reaction process was monitored by TLC dot plate. After the reaction was observed under UV light, the reaction flask was removed from the oil Lift in a bath, cool to room temperature, extract with ethyl acetate / water system to obtain ethyl acetate layer, concentrate under reduced pressure to obtain crude product, and finally use ethyl acetate / petroleum ether solution with a volume ratio of 1:2 as elution Reagent, silica gel column chromatography to obtain 170mg of light yellow oily liquid, yield 88%, 1 H NMR(600MHz, CDCl 3 )δ7.59(d,J=1.8Hz,1H), 7.58(s,1H), 7.42(m,J=2.4Hz,1H), 7.41(d,J=1.8Hz,1H), 4.93(s, 1H), 4.78 (d, J = 6.0 Hz, 1H), 4.60 (d, J = 6.0 ...
Embodiment 3
[0029] Example 3: Synthesis
[0030] Take the yellow powdery solid 4-methyl-nitrostyrene (100mg) into a 50mL round bottom flask, then add 10mL DMF as a solvent to it, and then add 1-methyl-2,3-O-isopropylene -5-azido-D-ribofuranose (156mg), copper chloride (5mg) as a catalyst, react at 100℃, TLC dot plate to monitor the reaction process, after the completion of the reaction is observed under ultraviolet light, remove the reaction flask from Lift in an oil bath, cool to room temperature, extract with ethyl acetate / water system to obtain ethyl acetate layer, concentrate under reduced pressure to obtain crude product, and finally use ethyl acetate / petroleum ether solution with a volume ratio of 1:2 as washing Removal of the agent, silica gel column chromatography to obtain 180mg of light yellow oily liquid, the yield is 85%, 1 H NMR(600MHz, CDCl 3 )δ7.63(s,1H), 7.24(d,J=3.0Hz,4H), 4.87(s,1H), 4.83(d,J=6.0Hz,1H), 4.56(d,J=6.0Hz, 1H), 4.52-4.49 (m, 1H), 4.39 (m, 2H), 3.20 (s, 3H), 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com