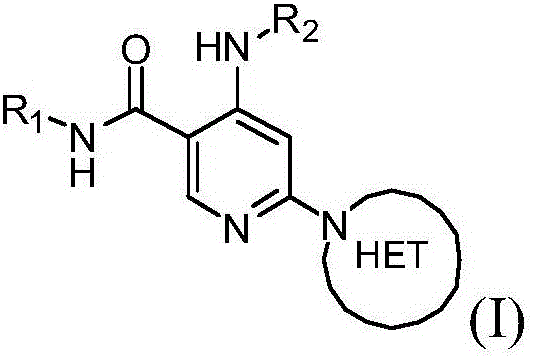
Heteroaryl substituted nicotinamide compounds
A technology of compounds and substituents, applied in the field of nicotinamide compounds, can solve problems such as signal transduction loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 133
[0405] Molecule / asymmetric unit of Example 133: 2
[0406]
[0407] Density (calculated) = 1.308g / cm 3 ,
[0408] wherein the unit cell parameters of Form N-1 are measured at a temperature of about 296K.
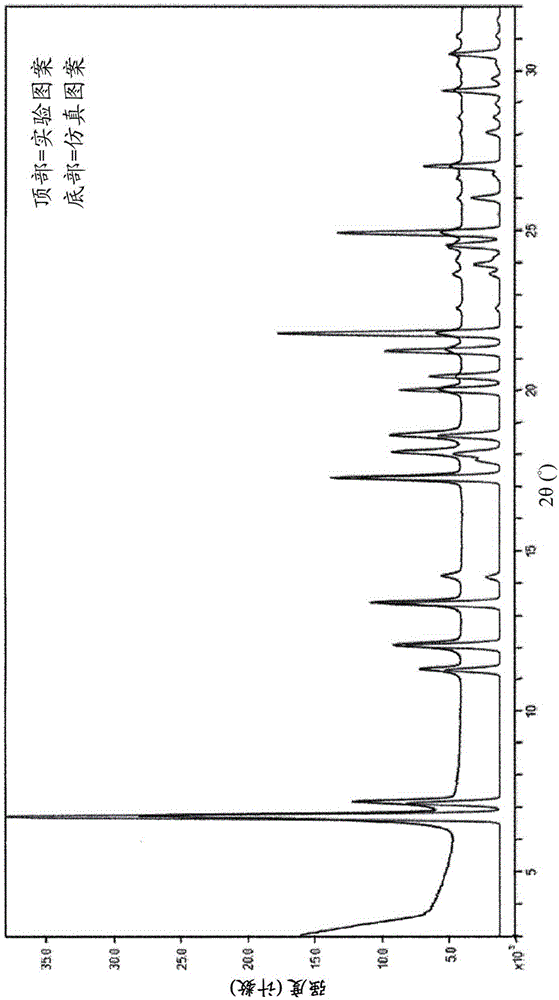
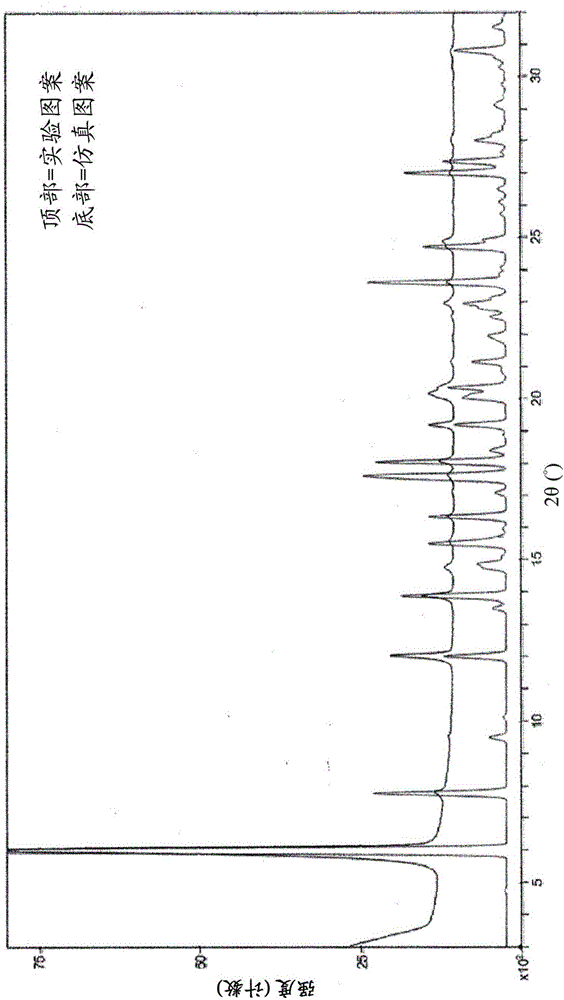
[0409] In another embodiment, the N-1 form of Example 133 is characterized as being substantially the same as figure 1 Simulated powder X-ray diffraction (PXRD) pattern consistent with the pattern shown.
[0410]In another embodiment, Form N-1 of Example 133 is characterized by fractional atomic coordinates substantially as set forth in Table 2.
[0411] Table 2
[0412] Fractional Atomic Coordinates Calculated at 296K for Form N-1 of Example 133
[0413] Atomic coordinates (×10 4 )
[0414] atom
X
Y
Z
atom
X
Y
Z
N(3)
6597
7363
4376
C(4)
6728
10155
3329
N(6)
6232
-333
7366
C(5)
5892
8650
2862
N(2)
5802
7040
3471
C(3)
7161
12160
3061
...
Embodiment 1
[0708] (R)-6-(5-cyano-1H-indol-1-yl)-N-(2-fluoro-3-hydroxy-3-methylbutyl)-4-(isopropylamino)nicotine Amide
[0709]
[0710] Step 1: To a solution of ethyl 4,6-dichloronicotinate (10 g, 45 mmol) in DMA (40 mL) was added propan-2-amine (5.3 g, 91 mmol) and DIPEA (31.7 mL, 182 mmol). The reaction mixture was stirred at room temperature for 48 h. The reaction mixture was diluted with MTBE and extracted with water (3x). The organic layer was washed with Na 2 SO 4 Dry, filter and concentrate to give crude product. The product was purified by flash chromatography over silica gel (10% EtOAc:petroleum ether as eluent) to afford ethyl 6-chloro-4-(isopropylamino)nicotinate (8.3 g, 75% yield) as a crystalline solid. LCMS m / z 243.7 (M+H); 1 H NMR (400MHz, DMSO-d 6 )δ8.54(s,1H),7.98(d,J=7.6Hz,1H),6.85(s,1H),4.29(q,J=7.2Hz,2H),3.86(m,1H),1.32( d, J=6.8Hz, 3H), 1.20(s, 3H), 1.19(s, 3H).
[0711]
[0712] Step 2: To a solution of ethyl 6-chloro-4-(isopropylamino)nicotinate (7 g...
Embodiment 1
[0716] To (R)-6-chloro-N-(2-fluoro-3-hydroxy-3-methylbutyl)-4-(isopropylamino)nicotinamide (100mg, 0.315mmol) and 1H-indole- To a solution of 5-carbonitrile (44.7 mg, 0.315 mmol) in 1,4-dioxane (5 mL) was added Cs 2 CO 3 (308mg, 0.944mmol) and Xantphos (72.8mg, 0.126mmol). Fill the reaction vessel with N 2 Purge for 20min, then add Pd 2 (dba) 3 (115 mg, 0.126 mmol) and purged again for 5 min. The vessel was heated at 110° C. in a sealed tube for 18 h. The reaction mixture was cooled to room temperature, via Filter and dilute with EtOAc (50 mL). The crude mixture was washed with water (10 mL) and brine (10 mL), then washed with Na 2 SO 4 dry. Concentration of the filtered solution gave crude compound, which was purified via silica gel chromatography (50% EtOAc in hexanes) to afford (R)-6-(5-cyano-1H-indol-1-yl)- N-(2-Fluoro-3-hydroxy-3-methylbutyl)-4-(isopropylamino)nicotinamide (20 mg, 15% yield). LCMS 424.2(M+H) + ; 1 H-NMR (400MHz, DMSO-d 6 )δ8.75(t, J=5.60Hz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com