Timosaponin aⅢ nano liposome and its preparation method and application
A nano-liposome and timosaponin technology, which is applied in the field of medicine and traditional Chinese medicine preparations, can solve the problems of affecting the efficacy of timosaponin AⅢ, low bioavailability, poor water solubility of timosaponin AⅢ, etc. The effect of drug action time, improvement of in vivo absorption, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
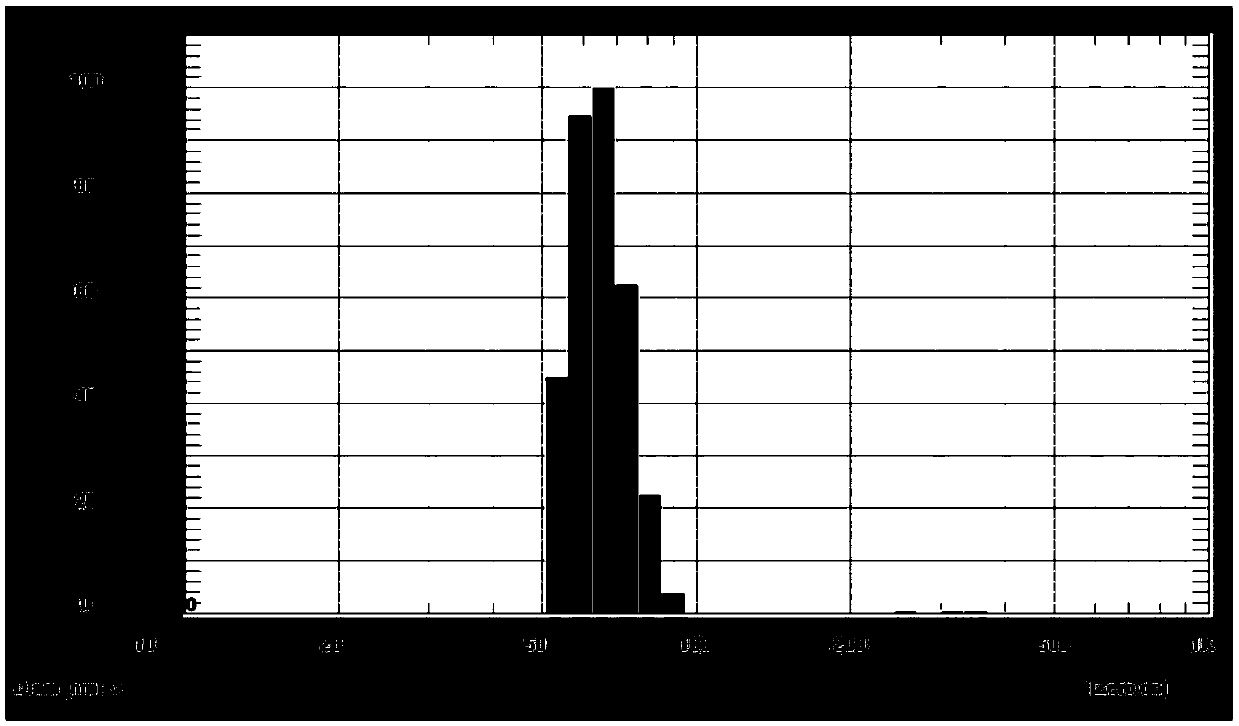
[0038] Embodiment 1, timosaponin AⅢ nano liposome preparation (prescription 1)
[0039]Weigh 0.8 mg timosaponin AⅢ and dissolve in 400 μL methanol; weigh 8.35 mg egg yolk lecithin (EPC) and dissolve it in 200 μL chloroform solution, and ultrasonically dissolve. Combine the above solutions into a round-bottomed test tube with a stopper, blow dry with nitrogen to remove methanol and chloroform, until a uniform lipid film is formed on the wall of the round-bottomed test tube. The molar ratio of EPC to timosaponin AⅢ was 10:1. -0.09MPa, 45~50℃, dry under reduced pressure to remove residual solvent and moisture, add 200μL 0.01mol / L phosphate buffer (pH=7.4), hydrate under 45~50℃ water bath for 1 hour, and obtain timosaponin AⅢ ester Plastid suspension. The timosaponin AⅢ liposome suspension is sonicated 3 times under the conditions of ultrasonic frequency 40-100 Hz and power 200-300 W, each ultrasonic time is 3-5 min, to obtain timosaponin AⅢ nano liposome.
Embodiment 2
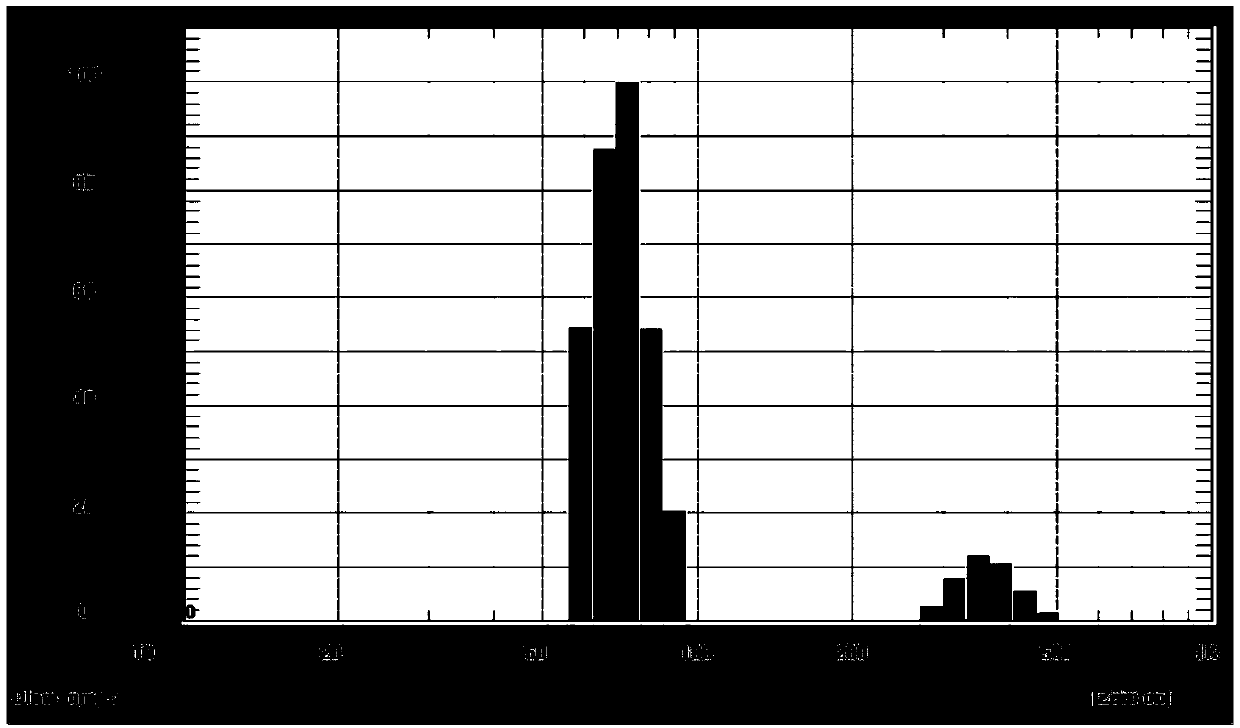
[0040] Embodiment 2, timosaponin AⅢ nano liposome preparation (prescription 2)
[0041] Weigh 0.8 mg timosaponin AⅢ and dissolve in 400 μL methanol, weigh 8.53 mg distearoylphosphatidylcholine (DSPC) and dissolve in 200 μL chloroform solution, and dissolve by ultrasonication. Combine the above solutions into a round-bottomed test tube with a stopper, blow dry with nitrogen to remove methanol and chloroform, until a uniform lipid film is formed on the wall of the round-bottomed test tube. The molar ratio of DSPC to timosaponin AⅢ was 10:1. -0.09MPa, 45~50℃, dry under reduced pressure to remove residual solvent and moisture, add 200μL 0.01mol / L phosphate buffer (pH=7.4), hydrate under 45-50℃ water bath for 1 hour, and obtain timosaponin AⅢ ester Plastid suspension. The timosaponin AⅢ liposome suspension is subjected to ultrasonication at an ultrasonic frequency of 40-100 Hz and a power of 200-300 W, and ultrasonicated 3 times for 3-5 minutes each time to obtain timosaponin AⅢ ...
Embodiment 3
[0042] Embodiment 3, timosaponin AⅢ nano liposome preparation (prescription 3)
[0043] Weigh 0.8 mg timosaponin AⅢ and dissolve in 400 μL methanol, weigh 7.51 mg egg yolk lecithin (EPC), 3.02 mg distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000) and dissolve in 500 μL chloroform solution , ultrasonically dissolved, combined the above solutions in a round-bottomed test tube, blown dry with nitrogen to remove methanol and chloroform, until a uniform lipid film was formed on the wall of the round-bottomed test tube. The molar ratio of EPC, DSPE-PEG2000 and timosaponin AⅢ was 9:1:1. -0.09MPa, 45~50℃, dry under reduced pressure to remove residual solvent and moisture, add 200μL 0.01mol / L phosphate buffer (pH=7.4), hydrate under 45-50℃ water bath for 1 hour, and obtain timosaponin AⅢ ester Plastid suspension. The timosaponin AⅢ liposome suspension is subjected to ultrasonication at an ultrasonic frequency of 40-100 Hz and a power of 200-300 W, and ultrason...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com