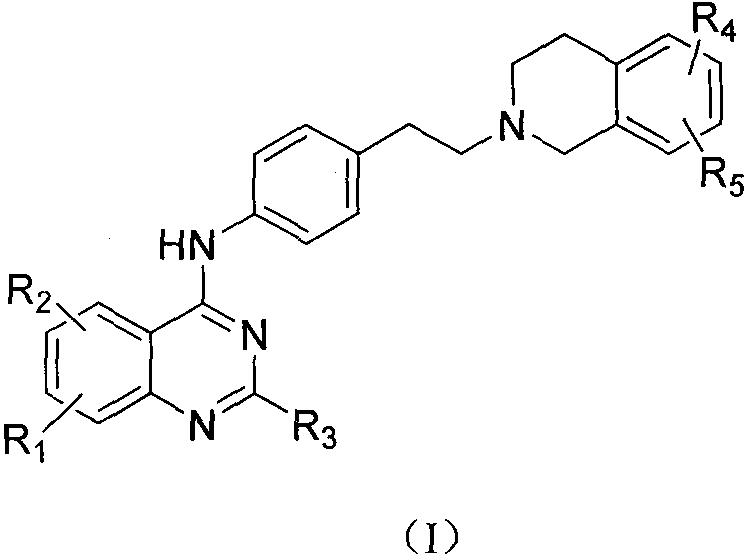
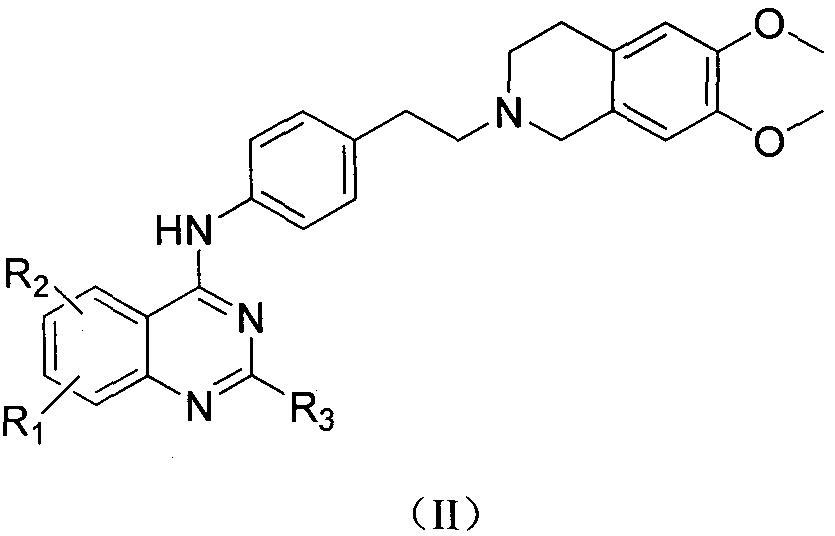
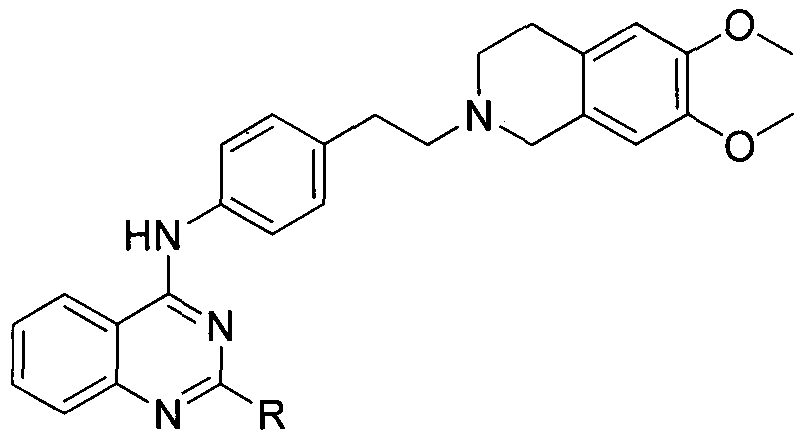
Quinazoline-N-phenethyl tetrahydroisoquinoline compound and preparation method and application thereof
A technology of dihydroisoquinoline and compound, applied in organic chemistry, drug combination, pharmaceutical formulation, etc., can solve the problems of limited application, large cardiovascular side effects, affecting plasma pharmacokinetics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Preparation of compound (i)
[0070] Under ice bath, freshly prepared aromatic acid chloride (20mmol) was slowly added to a solution of substituted or unsubstituted anthranilic acid (30mmol) in pyridine (15ml), stirred under ice bath for 30min, and then continued to stir at room temperature for 12h. The reaction solution was poured into water (200ml), stirred vigorously until a large amount of solids were precipitated, left to stand, filtered with suction, washed with water (20ml×5), and dried to obtain the product (i) as a white solid.
Embodiment 2
[0072] Preparation of Compound (ii)
[0073] Add the prepared compound (i), ammonia water (15ml) and ethanol (20ml) into a pressure-resistant bottle, seal it, heat to 80°C for 12h, cool, filter with suction, wash with water (15ml×3), and dry to obtain a white Solid product (ii).
Embodiment 3
[0075] Preparation of compound (iii)
[0076] The prepared compound (ii) (1 equiv) was added to thionyl chloride (10 equiv), and after adding a catalytic amount of DMF, it was heated to 50° C. for 6 h. Excess thionyl chloride was distilled off under reduced pressure to obtain a light yellow solid residue. Add the residue to an appropriate amount of 1N sodium hydroxide solution, stir and add an equal volume of dichloromethane for extraction, wash the organic layer with water and saturated brine, dry over anhydrous sodium sulfate, filter with suction, and evaporate under reduced pressure The product (iii) is obtained as a white or pale yellow solid after solvent removal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com