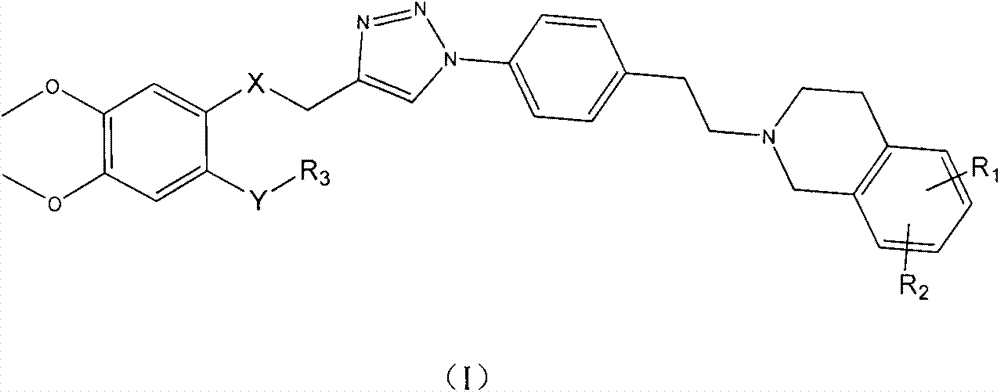
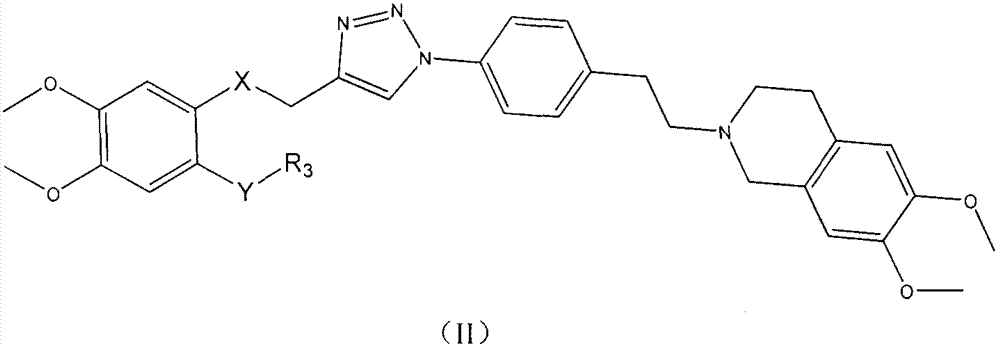
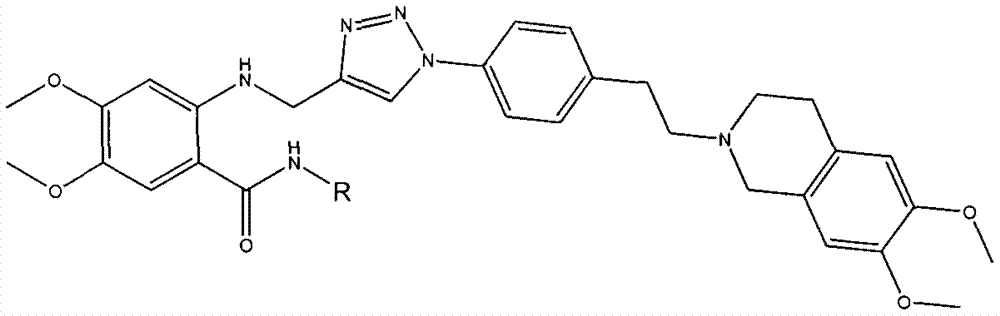
Triazole-N-phenethyl tetrahydronaphthalene isoquinoline compounds and, preparation method and application thereof
A technology of dihydroisoquinoline and compound, which can be used in organic chemistry, drug combination, antitumor drugs, etc., and can solve problems such as large cardiovascular side effects and limited application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Preparation of 6,7-dimethoxy-2-(4-nitrophenethyl)-1,2,3,4-tetrahydroisoquinoline (a)
[0072] Add p-nitrophenethyl bromide (2.42g, 10mmol), 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (2.41g, 10.5 mmol), anhydrous potassium carbonate (3.48g, 25.2mmol), acetonitrile 50ml. After heating to reflux for 18 hours, cool, filter, and wash the filter cake with dichloromethane. The filtrate was distilled under reduced pressure to remove the solvent to obtain a yellow solid. The solid was recrystallized from ethanol to obtain 2.76 g of a yellow needle-like crystalline solid with a yield of 81.2%.
[0073] 1H NMR (DMSO-d6, 300MHz) δ: 8.16-8.13 (d, J=8.7Hz, 2H), 7.57-7.53 (d, J=8.7Hz, 2H), 6.65(s, 1H), 6.62(s, 1H), 3.69(s, 6H), 3.53(s, 2H), 3.00-2.94(t, J=6.9Hz, 2H,), 2.75-2.68(m, 6H).
Embodiment 2
[0075] Preparation of 4-(2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)aniline (b)
[0076] Add compound a (11.02g, 32.2mmol), ethanol / dichloromethane mixed solvent (1:1, 60mL), Pd / C (0.58g) into a 100ml single-necked bottle, and hydrogen reduction reaction at room temperature for 48h. Diatomaceous earth was used as a bedding layer for filtration, and the filter cake was washed with dichloromethane. The filtrate was evaporated under reduced pressure to remove the solvent to obtain a light yellow solid, which was recrystallized from dichloromethane / petroleum ether to obtain 7.9 g of off-white solid, with a yield of 79.0%.
[0077] 1H NMR (300MHz, CDCl3) δ: 7.05-7.01 (d, J=8.1Hz, 2H), 6.65-6.62 (d, J=8.1Hz), 6.60(s, 6H), 3.64(s, 2H), 3.57 (s, 2H), 2.85-2.68 (m, 8H).
Embodiment 3
[0079]2-(((1-(4-(2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2 yl)ethyl)phenyl)-1H-1,2,3 Preparation of -triazol-4-yl)methyl)amino)phenyl)-4,5-dimethoxy-N-benzanilide (1)
[0080] (a) Preparation of 2-(4-azidophenethyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (c)
[0081] Compound b (4.8g15.36mmol) was dissolved in 30ml of 50% acetic acid, and sodium nitrite (1.38g19.97mmol) was slowly added dropwise at 0-5°C, keeping the temperature constant, and vigorously stirred for 50min. At 0-5°C, sodium azide (1.40 g, 21.51 mmol) was added in batches, and the temperature was kept constant, and stirring was continued for 1 h. The reaction solution was poured into 200ml of ice water, extracted with ethyl acetate (3*100mL), the organic layer was washed with water (3*60mL), saturated sodium bicarbonate (3*60mL), and saturated brine (3*50mL), Finally, anhydrous sodium sulfate was added for drying. The solvent was removed by distillation under reduced pressure to obtain 3.3 g of pink soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com