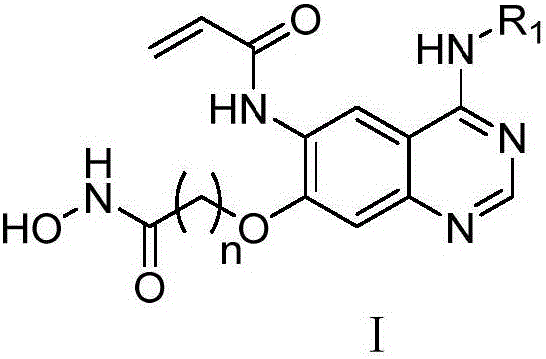
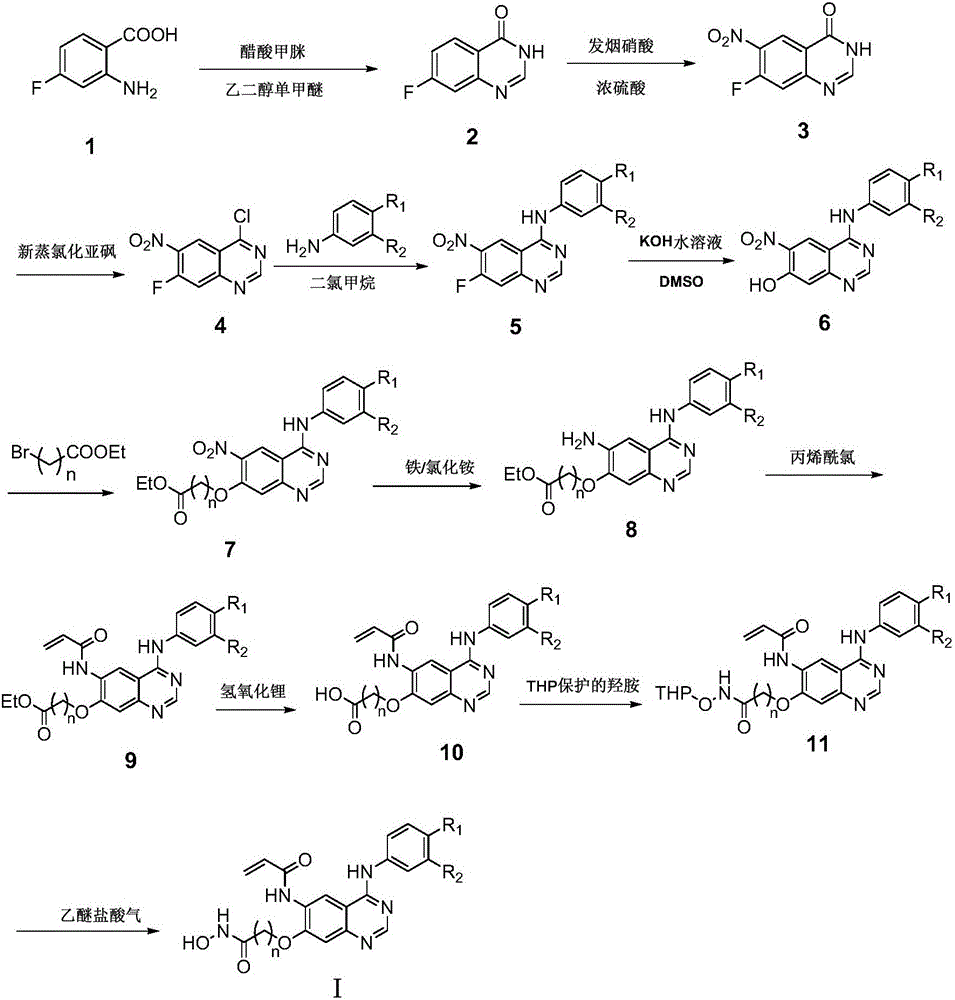
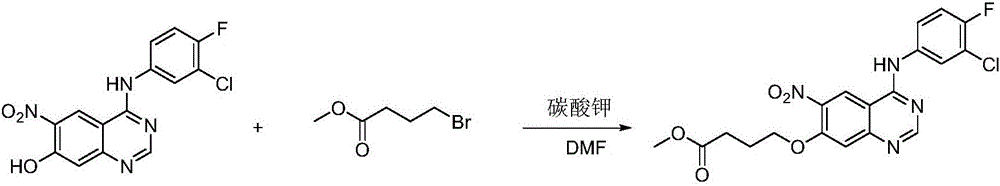
Quinazoline derivatives containing hydroxamic acid side chain as well as preparation and application thereof
A technology of hydroxamic acid and quinazoline, applied in the field of quinazoline derivatives, can solve the problems of lung cancer treatment obstacles and the like, and achieve the effects of enhancing anti-drug resistance, preventing desensitization, and good inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 4-(6-acrylamido-4-(3-chloro-4-fluoroanilino)quinazolin-7-yl)oxy-N-hydroxybutanamide;
[0032] step:
[0033] Raw material 1: 4-(3-chloro-4-fluoroanilino)-6-nitro-7-hydroxyquinazoline was prepared according to the method of document CN201210411352.7.
[0034] Raw material 2: Preparation of ethyl 4-(4-(3-chloro-4-fluoroanilino)-6-nitroquinazolin-7-yl)oxybutyrate:
[0035]
[0036] Dissolve 4-(3-chloro-4-fluoroanilino)-6-nitro-7-hydroxyquinazoline (1.1 g, 3.3 mmol) and ethyl 4-bromobutyrate (3.3 mmol) in 10 mL of DMF , Potassium carbonate (0.45g, 3.3mmol) was added to the mixed system, and the reaction was stirred at room temperature for 36 hours. After the reaction was completed, a large amount of solid was precipitated by adding water, and the product was collected by suction filtration and purified by column chromatography.
[0037] 1 H NMR(500MHz,DMSO)δ9.58(s,1H),8.93(s,1H),8.54(s,1H),8.14(d,J=4.8Hz,1H),7.91–7.65(m,1H) ,7.43(t,J=9.0Hz,1H),7.28(s,1H),4....
Embodiment 2
[0059] Example 2 4-(6-acrylamido-4-(3-bromoanilino)quinazolin-7-yl)oxy-N-hydroxybutanamide
[0060] With reference to the method of Example 1, only 4-((6-acrylamido-4-(3-chloro-4-fluoroanilino) quinazoline-7-yl) oxy)-N-((tetrahydro -2H-pyran-2-yl)oxy)butanamide is replaced by 4-((6-acrylamido-4-(3-bromoanilino)quinazolin-7-yl)oxy)-N- ((Tetrahydro-2H-pyran-2-yl)oxy)butanamide, 88.3% yield. m.p.:200-202℃; 1 H-NMR(500MHz,DMSO)δ11.34(s,1H),10.60(s,1H),9.69(s,1H),9.28(s,1H),8.90(s,1H),7.96(t,J =1.9Hz,1H),7.71–7.68(m,1H),7.53–7.49(m,1H),7.45(m,2H),6.97(dd,J=17.0,10.2Hz,1H),6.37(dd, J=17.0,1.7Hz,1H),5.87(dd,J=10.2,1.7Hz,1H),4.27(t,J=5.8Hz,2H),2.27(t,J=6.7Hz,2H),2.18– 2.11(m,2H).HRMS(ESI)m / z calcdfor C 21 h 20 BrN 5 o4 [M+H] + :484.0872. Found: 484.0873.
Embodiment 3
[0061] Example 3 4-(6-acrylamido-4-(3-chloro-4-fluoroanilino)quinazolin-7-yl)oxy-N-hydroxycaproamide
[0062] With reference to the method of Example 1, only 4-((6-acrylamido-4-(3-chloro-4-fluoroanilino) quinazoline-7-yl) oxy)-N-((tetrahydro -2H-pyran-2-yl)oxy)butanamide was replaced by 4-((6-acrylamido-4-(3-chloro-4-fluoroanilino)quinazolin-7-yl)oxy )-N-((tetrahydro-2H-pyran-2-yl)oxy)pentanamide, yield 91.3%.
[0063] m.p.:174-176℃; 1 H-NMR(500MHz,DMSO)δ11.49(s,1H),10.58(s,1H),9.93(s,1H),9.21(s,1H),8.91(s,1H),7.96(dd,J =6.8,2.6Hz,1H),7.67(ddd,J=8.9,4.3,2.6Hz,1H),7.55(m,2H),6.85(dd,J=17.0,10.2Hz,1H),6.36(dd, J=17.0,1.8Hz,1H),5.87(dd,J=10.3,1.7Hz,1H),4.27(t,J=6.3Hz,2H),2.09(t,J=7.2Hz,2H),1.94– 1.81(m,2H),1.78–1.68(m,2H).HRMS(ESI)m / z calcd for C 22 h 21 ClFN 5 o 4 [M+H] + :472.1439. Found: 472.1435.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com