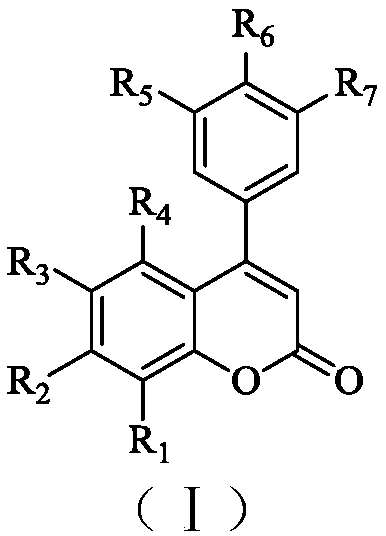
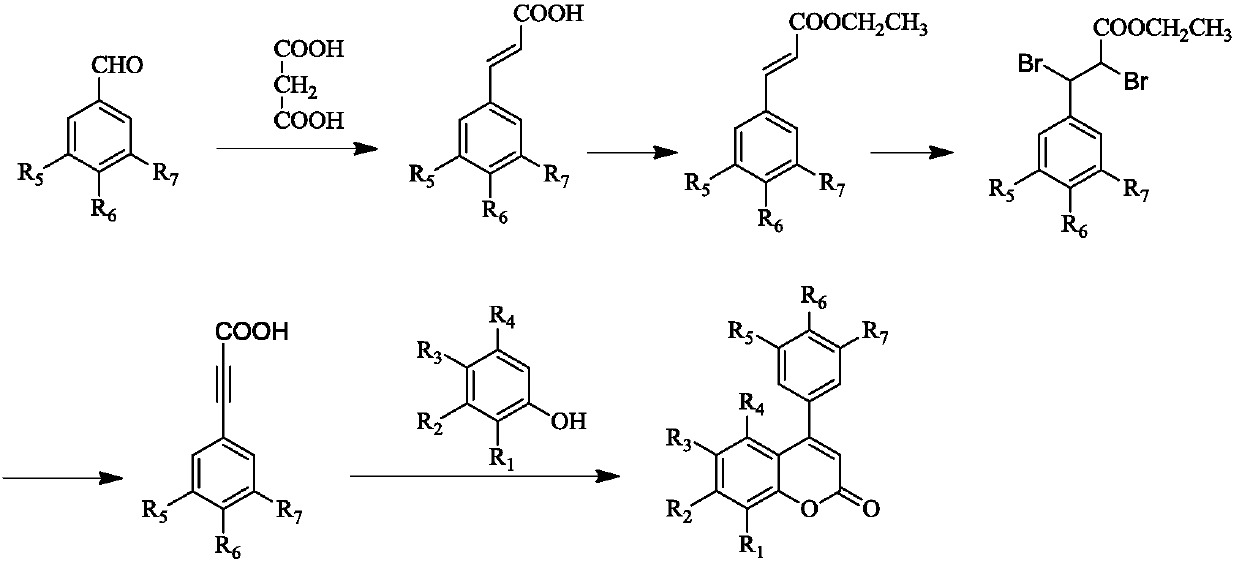
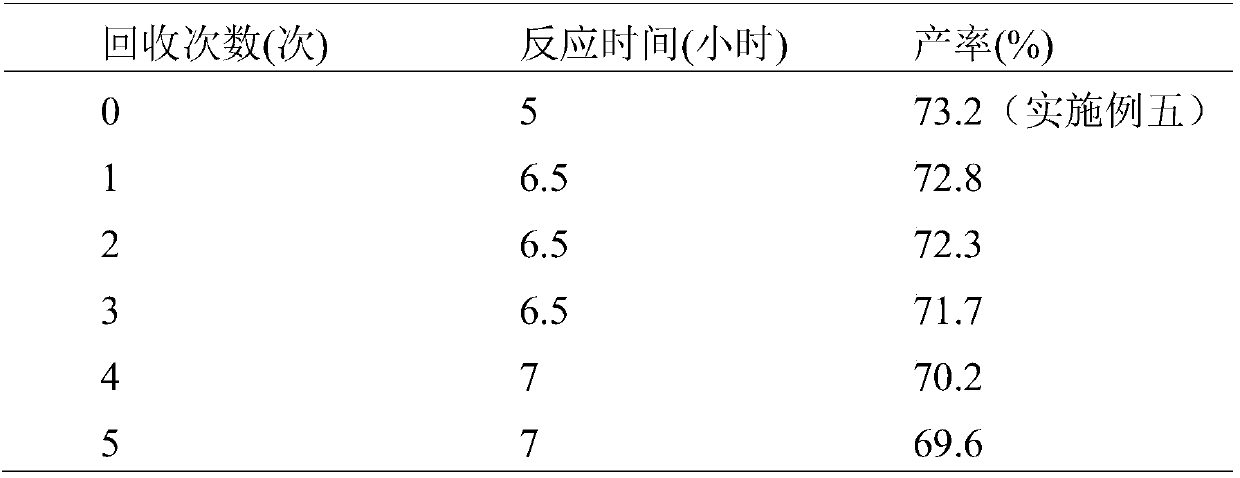
A kind of preparation method of 4-aryl coumarin compound
An aryl coumarin and compound technology, which is applied in the field of preparation of 4-aryl coumarin compounds, can solve the problems of inability to recycle, long reaction time, poisonous catalyst, etc., and achieves short reaction time, simple operation, Ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment one: the preparation of acidified montmorillonite K-10
[0063] Add 13g of montmorillonite K-10 to 130ml of sulfuric acid solution (mass concentration: 30%) to make a suspension, heat and reflux at 90-100°C for 4 hours, filter with suction, wash with distilled water until neutral, and vacuum at 40°C After drying, 12.95 g of acidified montmorillonite K-10 was obtained.
Embodiment 2
[0064] Example 2: Preparation of 4-(4'-methoxyphenyl)-7-hydroxycoumarin
[0065] 1. Add 25g (240mmol) malonic acid, 24ml (197.6mmol) p-methoxybenzaldehyde, 40ml (496mmol) pyridine, 2ml (20.4mmol) hexahydropyridine into a 100ml three-necked reaction flask, and heat to 75°C Reflux for 7 hours, complete the reaction, cool for 10 minutes, add 150ml of 3mol / ml glacial hydrochloric acid solution, let stand overnight, filter with suction, and wash the precipitate with 1000ml of water to obtain a white crude product. The crude product was recrystallized from absolute ethanol to obtain 31.2 g of white pure product 4-methoxyphenylacrylic acid, with a yield of 97.5%.
[0066] 2. Add 7.12g (40mmol) 4-methoxyphenylacrylic acid and 100ml (1717mmol) absolute ethanol to a 250ml three-necked reaction flask, add 4.4ml (60mmol) thionyl chloride dropwise, heat to 82°C, and reflux the reaction 2 hours until the raw material point completely disappeared, stop the reaction and cool for 10 minutes, ...
Embodiment 3
[0074] Example 3: Preparation of 4-(4'-methoxyphenyl)-7,8-dihydroxycoumarin
[0075] In a 100ml three-necked reaction flask, add 1.76g (10mmol) 4-methoxyphenylpropiolic acid, 1.39g (11mmol) pyrogallol, 20ml (196mmol) nitrobenzene, stir well, and heat to 100°C , then add 4g of sulfuric acid acidified montmorillonite K-10, TLC monitors the reaction progress (developing agent: dichloromethane:methanol=10:1), after 7 hours the reaction is complete, filter while hot, add 30ml petroleum ether in the filtrate, Stand overnight for natural crystallization, filter with suction, wash the precipitate with 20 ml of petroleum ether, and dry in a vacuum oven to obtain 1.9 g of white pure product with a yield of 66.9%.
[0076] IR: 3165(OH); 1685(C=O); 2971,1602,1560,1508,1442,1114,1043,1017,836(benzene ring); 1241,1177(C-O); 2841(-OCH 3 ).
[0077] 1 H NMR (600MHz, DMSO-d 6 )δ:3.84(s,3H),6.09(s,1H),6.80(d,J=8.8Hz,1H),6.85(d,J=8.7Hz,1H)7.10(d,J=8.7Hz,2H ), 7.46(t, J=5.7Hz, 2H), 9.42(s, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com