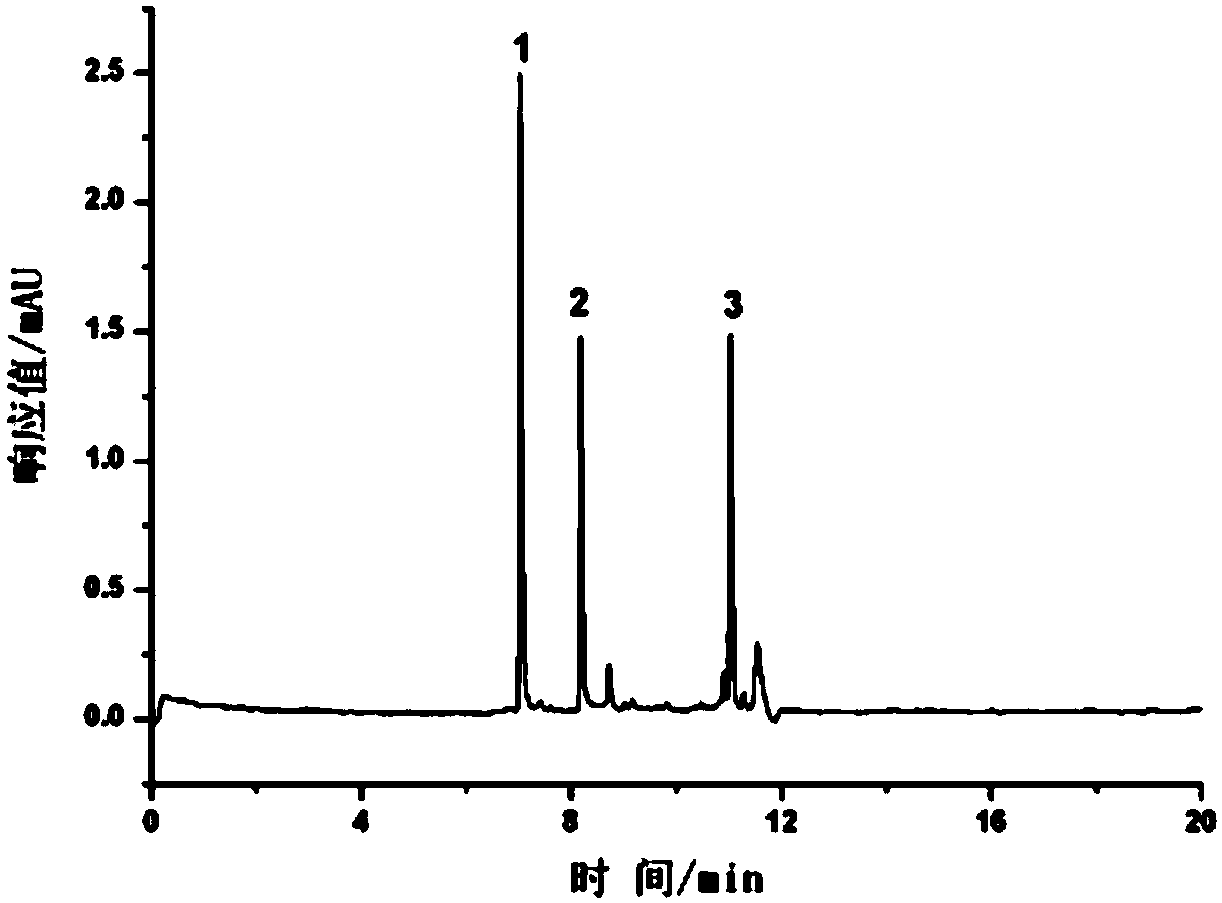
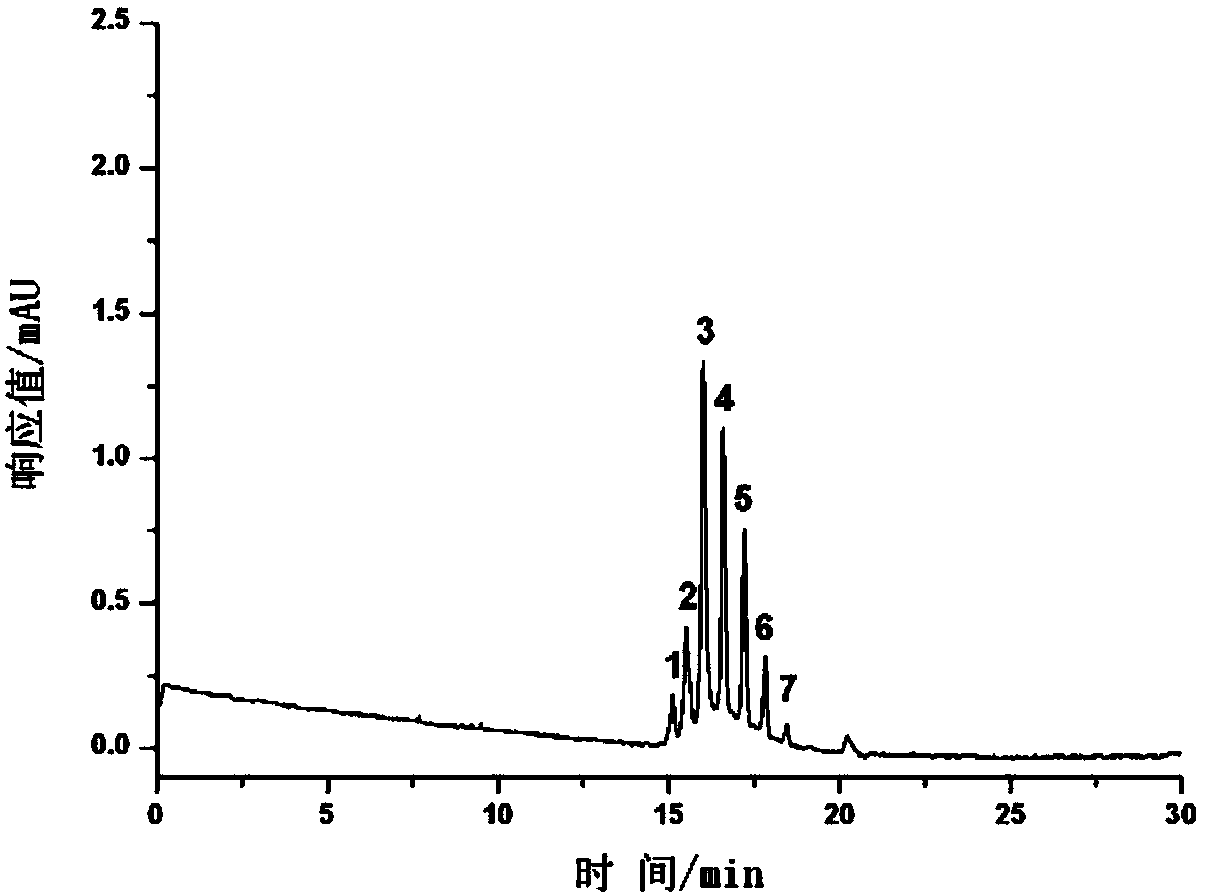
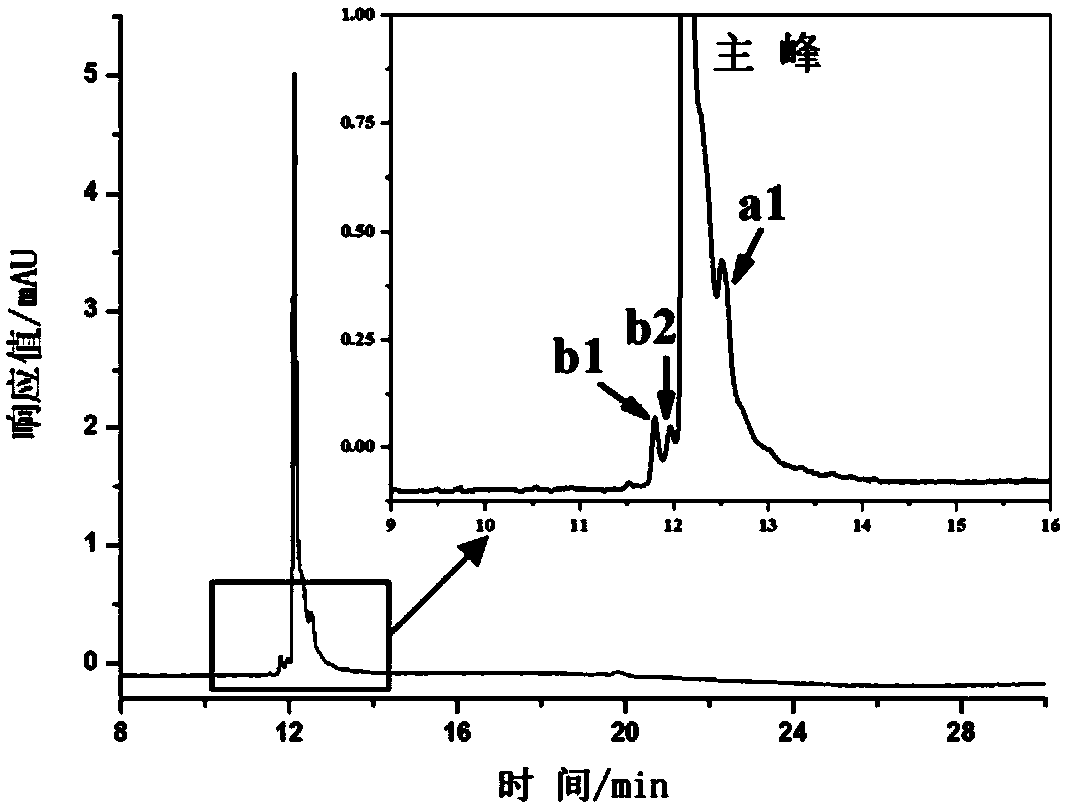
Protein Modified Open-Tube Column and Its Application in the Separation of Monoclonal Antibody Charge Variant
A protein separation and open-column technology, which is applied in the field of instrumental analysis, can solve problems such as difficulties in the characterization of monoclonal antibodies, and achieve the effects of improving the separation effect, increasing the separation selectivity, and inhibiting the adsorption of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The open-tube column modified by bovine serum albumin (BSA) is prepared by the following steps:
[0040] (1) Preparation of PDDA open-tube column: Dissolve PDDA and sodium chloride into 20mM Tris-HCl (pH 8.3) to obtain a PDDA solution with a PDDA concentration of 20mg / mL, wherein the salt ion concentration is 0.1M, and then Flush the capillary column for 1 h at a flow rate of 10 μL / min. Finally, remove excess PDDA solution with deionized water.
[0041] (2) Preparation of PDDA@BSA open-tubular column: After successful preparation of PDDA open-tubular column, take BSA and add 20mM Tris-HCl (pH 7.4) to make a 5mg / mL homogeneous solution, and then mix it with 10μL / min The flow rate was injected into the prepared PDDA open-tube column, and it was kept in an oven at 40° C. for 12 hours. Finally, the excess BSA was removed with deionized water, and dried under nitrogen protection at 40 °C to obtain a PDDA@BSA open-tube column.
Embodiment 2
[0043] The open-tube column modified by bovine serum albumin (BSA) is prepared by the following steps:
[0044] (1) Preparation of PDDA open-tube column: get PDDA and potassium chloride dissolved in 20mM Tris-HCl (pH value 8.3) to obtain a PDDA solution with a PDDA concentration of 10mg / mL, wherein the salt ion concentration is 1.0M, and then Flush the capillary column for 0.5 h at a flow rate of 10 μL / min. Finally, remove excess PDDA solution with deionized water.
[0045] (2) Preparation of PDDA@BSA open-tubular column: After successful preparation of PDDA open-tubular column, take BSA and add 20mM Tris-HCl (pH 7.4) to make a 10mg / mL homogeneous solution, and then prepare it at 10μL / min The flow rate was injected into the prepared PDDA open-tube column, and it was kept in an oven at 40° C. for 12 hours. Finally, the excess BSA was removed with deionized water, and dried under nitrogen protection at 40 °C to obtain a PDDA@BSA open-tube column.
Embodiment 3
[0047] The open-tube column modified by bovine hemoglobin (BHb) is prepared by the following steps:
[0048] (1) Preparation of PDDA open-tube column: Dissolve PDDA and sodium chloride into 20mM Tris-HCl (pH 8.3) to obtain a PDDA solution with a PDDA concentration of 20mg / mL, wherein the salt ion concentration is 2.0M, and then Flush the capillary column for 3 h at a flow rate of 10 μL / min. Finally, remove excess PDDA solution with deionized water.
[0049] (2) Preparation of PDDA@BHb open-tubular column: After successful preparation of PDDA open-tubular column, take BHb and add 20mM Tris-HCl (pH 7.4) to make a 5mg / mL homogeneous solution, and then prepare it at 10μL / min The flow rate was injected into the prepared PDDA open-tube column, and it was kept in an oven at 40° C. for 12 hours. Finally, excess BHb was removed with deionized water, and dried under nitrogen protection at 40 °C to obtain a PDDA@BHb open-tube column.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com