Reversible and photochromic phosphate material and preparation method thereof
A technology of photochromic materials and phosphates, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems of poor anti-oxidation performance, little research on materials, slow development speed, etc., and achieve good anti-fatigue properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The present invention also provides a method for preparing the above-mentioned phosphate reversible photochromic material, comprising: mixing Sr compound, Re 1 Compounds, phosphide-containing compounds, Eu-containing compounds, Mn-containing compounds and Re-containing 2 Compounds are mixed, pre-fired, and then fired in a weak reducing atmosphere to obtain a phosphate reversible photochromic material; Re 1 One of the rare earth elements Y or Lu; Re 2 is a rare earth element, and Re 1 with Re 2 Not the same element at the same time; the Sr compound, containing Re 1 Compounds, Eu-containing compounds, Mn-containing compounds and Re-containing 2 Sr, Re in the compound 1 , Eu, Mn and Re 2 The molar ratio is (9-x-y):(1-z):x:y:z; 0
[0052] Among them, the Re 2 , x, y and z are the same as above, and will not be repeated here.
[0053] In the present invention, there is no particular limitation on the sources of all raw materials, and commerci...
Embodiment 1
[0063] According to the following chemical composition: Sr 8.78 Y(PO 4 ) 7 :0.02Eu 2+ ,0.2Mn 2+ , Weigh strontium carbonate, yttrium oxide, ammonium dihydrogen phosphate, europium oxide and manganese carbonate respectively, mix and grind until uniform, put the resulting mixture in a corundum crucible, put it in a muffle furnace, and heat up to 600°C, keep warm for 4 hours, take it out after cooling to room temperature naturally, and grind it evenly again, then put it into a tube furnace and heat it up to 1300°C for 4 hours, while passing a weak reducing gas (volume ratio of N 2 :H 2 =80%:10%). Finally, after it is naturally cooled to room temperature, it is taken out and ground again to obtain a phosphate reversible photochromic material.
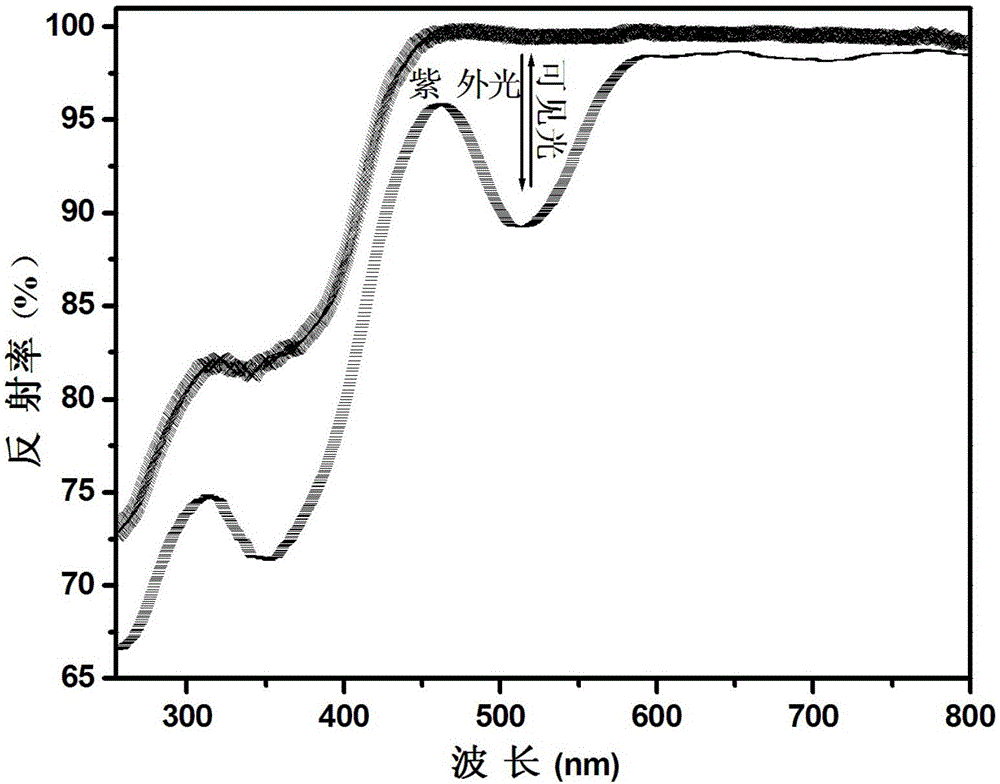
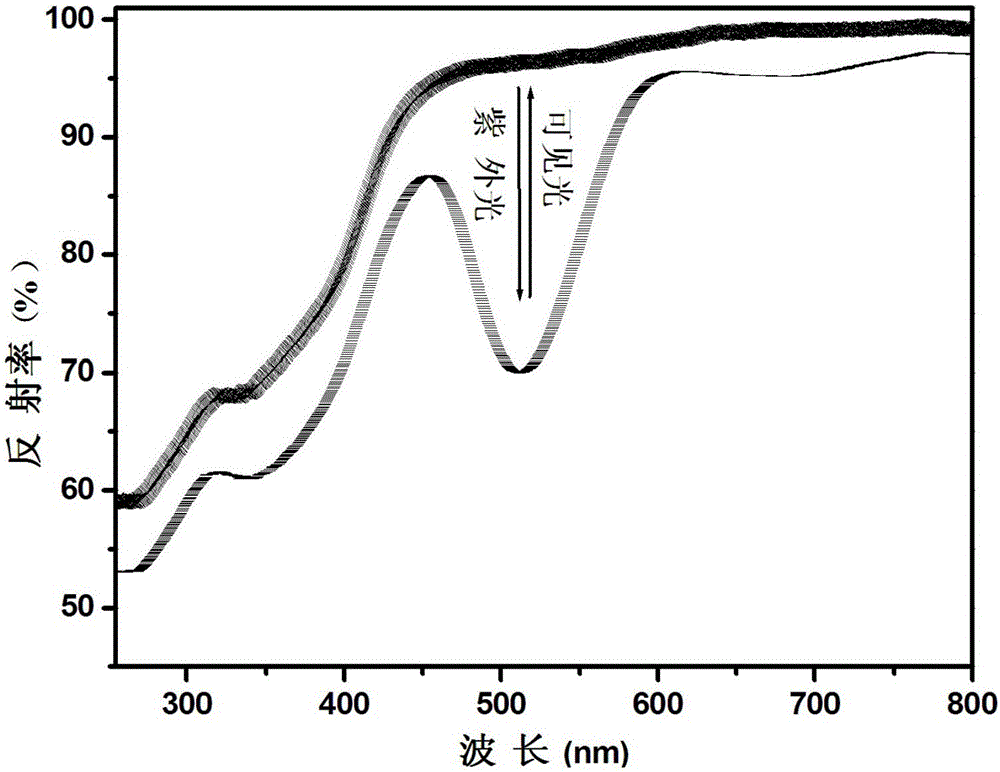
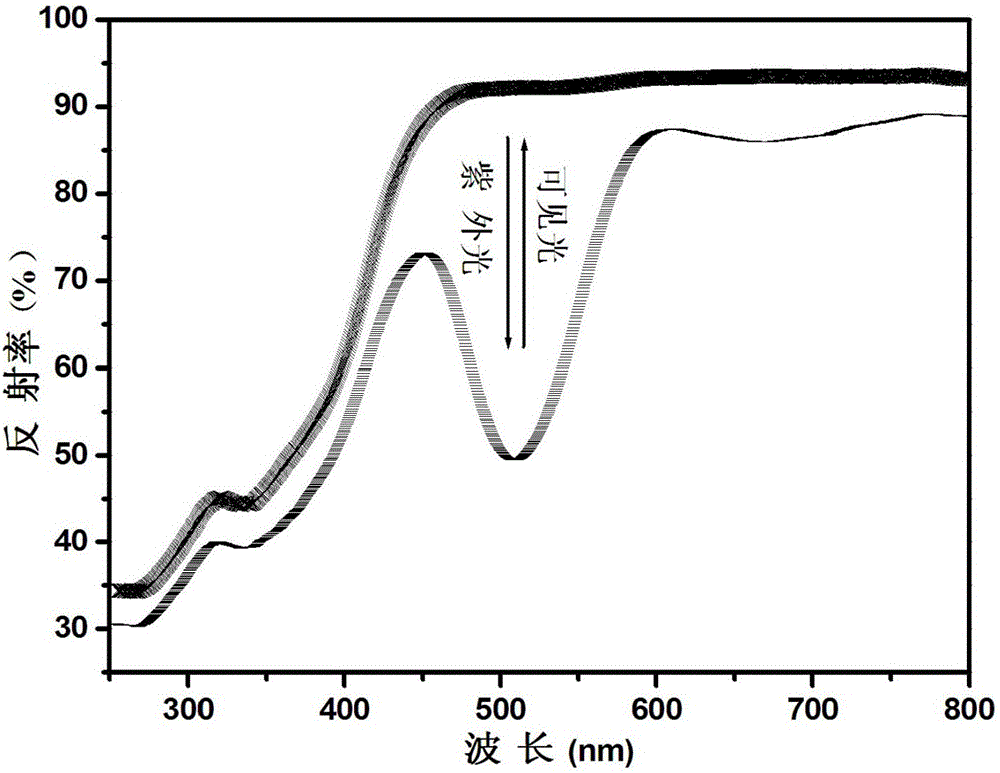
[0064] Utilize ultraviolet-visible spectrophotometer to analyze the phosphate reversible photochromic material obtained in embodiment 1, obtain its diffuse reflectance spectrogram after ultraviolet light and visible light alternate ir...
Embodiment 2
[0066] According to the following chemical composition: Sr 8.77 Y(PO 4 ) 7 :0.03Eu 2+ ,0.2Mn 2+ , Weigh strontium carbonate, yttrium oxide, ammonium dihydrogen phosphate, europium oxide and manganese carbonate respectively, mix and grind until uniform, put the resulting mixture in a corundum crucible, put it in a muffle furnace, and heat up to 500°C, keep warm for 5 hours, take it out after cooling to room temperature naturally, and grind it evenly again, then put it into a tube furnace and heat it up to 1350°C for 4 hours, while passing a weak reducing gas (volume ratio of N 2 :H 2 =80%:10%). Finally, after it is naturally cooled to room temperature, it is taken out and ground again to obtain a phosphate reversible photochromic material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com