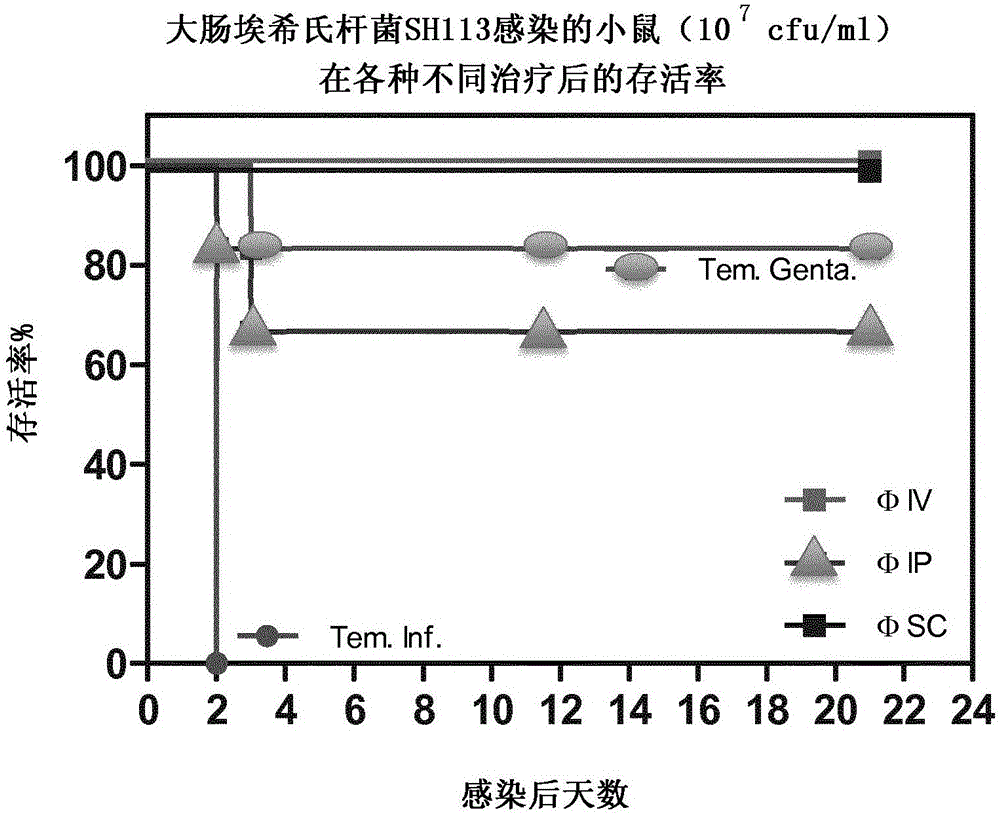
Phage therapy of E coli infections
A technology of Escherichia coli and bacteriophage, which is applied in the field of manufacturing, can solve the problem of pathogenic bacteria and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
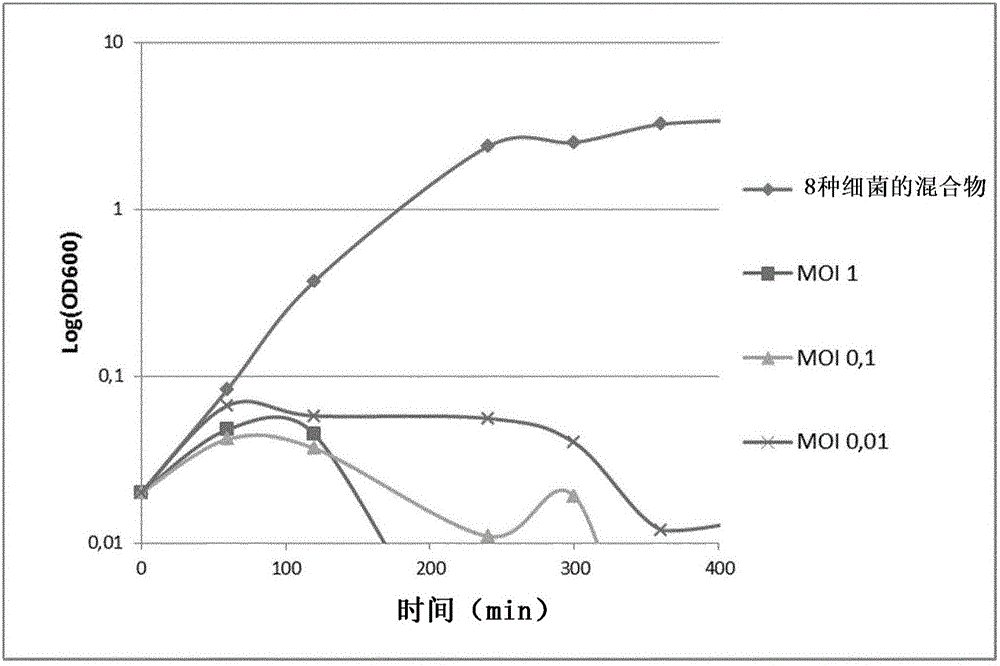
[0193] Example 1: Phage-host characteristics and kinetics
[0194] One-step growth experiments were performed as previously described to first determine productive lysis time, adsorption rate, and then phage release. To determine the rate of adsorption, samples were taken at different time intervals to analyze free phage particles in solution. For the determination of the productive time and the amount of phage released, E. coli bacteria were mixed with the phage solution and the phage were allowed to adsorb for 15 min. The mixture was immediately centrifuged at 5000 rpm for 10 min to remove free phage particles. The pellet was resuspended in 5 fresh LB medium and the culture was continued to incubate at 37°C. Samples were taken at 3 min intervals and phage titers determined. These results allowed calculation of the number of phage produced per bacterium (amount released), productive time and productive lytic effect (PLE), as shown in Table 3 below.
[0195] table 3
...
Embodiment 2
[0198] Example 2: Preparation of Mixed Compositions
[0199] The following mix compositions were constructed, each containing 10 -9 to 10 -11 Each phage between pfu:
[0200] Table 4
[0201] mixture Phage I BP953+BP1168 II BP953+BP1168+BP1229 III BP953+BP1151+BP1155+BP1176 IV BP700+BP953+BP970+BP1002+BP1176 V BP1002+BP1151+BP1155+BP1168+BP1176 VI BP539+BP700+BP753+BP814+BP1151+BP1176+BP1168
[0202] The following two other mixed compositions containing all the various phages covering the most important diversity of E. coli species were constructed.
[0203] Table 5A: Mix Composition A :
[0204]
[0205] Table 5B: Mix Composition B :
[0206]
Embodiment 3
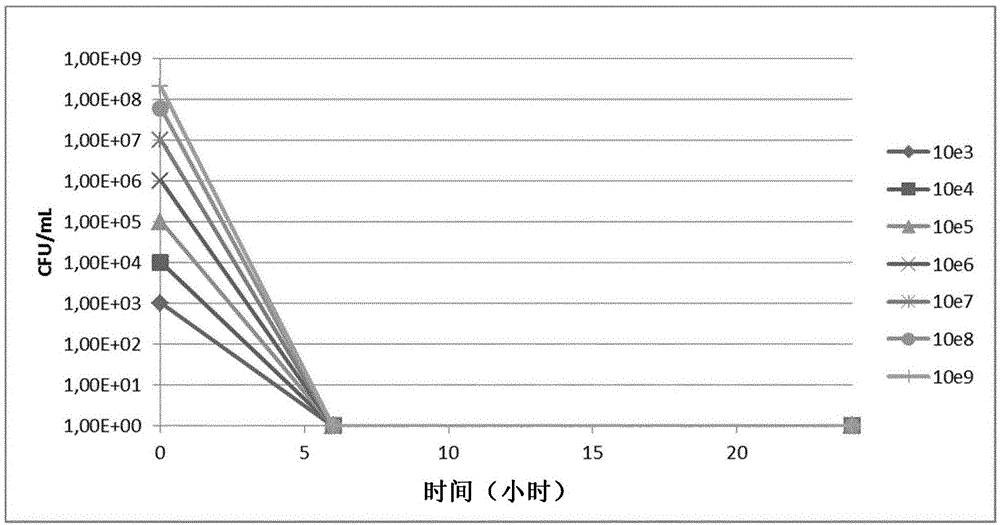
[0207] Example 3: Bacterial susceptibility to the phage mixture of the invention
[0208] Use the phage mixture of the present invention to 2.10 9 A concentration of phage / ml was tested on various bacterial strains. Different bacterial concentrations were plated at a concentration of 2.10 9 phage / ml phage mixture and incubated at 37°C for 24h.
[0209] The mixture was tested on the different Escherichia coli bacteria listed in Table 2 as well as additional Escherichia coli bacteria including meningitis-causing bacteria from the R. Debré collection. coli bacteria (37 strains), BLSE (5 strains) and ST131 (9 strains) types of Escherichia coli bacteria, Escherichia coli bacteria from hospitalized patients (35 strains) and O157, Hemorrhagic Escherichia coli bacteria of type 0144 and 0104 (3 strains). The % of bacterial species susceptible to the mixture is listed in Table 6 below:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com